Key Findings
>> HER2-targeted CAR T cells kill iPSC-derived cardiomyocytes in vitro.
Abstract
CAR T cells have emerged as a promising treatment option for several cancers. However, CAR T cell treatment often leads to deleterious side effects, such as on-target, off-tumor toxicity. It is therefore important to develop methods to screen for the side effects of CAR T cells prior to their use in the clinic. Here, we develop an in vitro model of on-target, off-tumor side effects of CAR T cells on the Maestro MEA platform.
Introduction
In recent years, chimeric antigen receptor T (CAR T) cells have become one of the most exciting and well-researched treatments for cancer. To create a CAR T cell, T cells are isolated and engineered to express a CAR that targets a specific antigen, often a cell surface marker of the cancer being targeted. Once the CAR engages with its target, the CAR T cells are activated and begin to kill the targeted tumor cells (Vormittag et al., 2018). This system has proven to be highly effective in eliminating tumor cells both in in vitro models and in the clinic (Aghajanian et al., 2022). However, CAR T cell therapy is currently hampered by off-tumor toxicity that can manifest in multiple forms and thwart clinical success. Following tumor killing in vivo, robust activation and expansion of CAR T cells can lead to marked increases of inflammatory cytokines such as IFN-γ, IL-2, IL-6, and IL-10, termed cytokine release syndrome (CRS) (Frey and Porter, 2019). Overproduction of these cytokines can lead to endothelial cell damage and subsequent endothelial leakage. The lack of adequate perfusion to the tissues following CRS can lead to organ dysfunctions including pulmonary edemas and arrythmias (Xiao et al., 2021).
While CRS can be considered an “off-target” consequence of CAR T cell therapy, other side effects can be the result of direct engagement of the target antigen. CAR T cells may attempt to kill healthy cells throughout the body that express their target antigen, leading to unwanted damage to non-cancerous tissue (Flugel et al., 2023). For example, CAR T cells targeting the receptor tyrosine kinase human epidermal growth factor 2 (HER2) are currently being investigated for use in breast cancer patients whose tumor cells overexpress HER2. However, HER2 is also expressed in other tissues in the body, including in cardiomyocytes (CMs) (Kitani et al., 2019). Following treatment, HER2-targeted CAR T cells could bind to CMs and induce cytotoxicity, leading to heart problems such as myocarditis, loss of contractile function, or arrythmias (Sendur et al., 2013; Totzeck et al., 2022).
To evaluate on-target, off-tumor effects, we developed an in vitro model using HER2-CAR T cells and induced pluripotent stem cell-derived CMs (iPSC-CMs). We measured both viability and electrophysiological changes in the iPSC-CMs in response to HER2-CAR T dosing using the Maestro Pro. Our data show that HER2-CAR T cells kill iPSC-CMs in both a dose-dependent and antigen-specific manner. Interestingly, we also observed electrophysiological changes in iPSC-CM cultures in response to HER2-CAR T cell dosing that often preceded changes in CM viability. This may suggest that treatment doses that do not induce off-tumor cell death may still produce functional side effects, highlighting the need for more sensitive methods to detect these functional changes. In total, our results demonstrate that the Maestro Pro can effectively measure the on-target, off-tumor effects of CAR T cells in an in vitro model.
Materials and Methods
Cells and reagents
iCell cardiomyocytes (CMs) (Cat. R1057) were obtained from FUJIFILM Cellular Dynamics (Madison, WI). iCell CM plating media (Cat. M1001) and maintenance media (Cat. M1003) were obtained from FUJIFILM Cellular Dynamics (Cat. R1057) and supplemented with 1% penicillin/streptomycin (Gibco, Cat. 15140122).
iCell glutaneurons were obtained from FujiFilm Cellular Dynamics (Cat. R1061). iCell neuron media was composed of BrainPhys Neuronal Medium (STEMCELL Technologies, Cat. 05790), N2 Supplement (Thermo-Fisher Scientific, Cat. 17502-048), penicillin/streptomycin (Gibco, Cat. 15140122), iCell Neural Supplement B (FUJIFILM Cellular Dynamics, Cat. M1029), iCell Nervous System Supplement (FUJIFILM Cellular Dynamics, Cat. M1031) and laminin (Matrixome, Cat. 892 011).
HER-2 SCFV-CD28-CD3ζ CAR T cells (HER2-CAR T) (Cat. PM- CAR1024) and HER2-CAR T cell media (Cat. PM-CAR2001) were obtained from ProMab Biotechnologies, Inc. (Richmond, CA). Pan T cells were obtained from Lonza Bioscience (Cat. 4W-350).
Flow cytometry
To stain iCell CMs for HER2 expression, iCell CMs were lifted from CytoView 24-well plates using 0.5% Trypsin/EDTA (Gibco, Cat. 15400-054) and centrifuged 200 x g for 5 minutes. CMs were then resuspended in 100 µL ice-cold 5% goat serum (Life Technologies, Cat. 50062Z) in phosphate buffered saline (PBS) (Gibco, Cat. 10010023). CMs were stained with an ErbB2/HER2 antibody (R&D Systems, Cat. MAB1129) at a final concentration of 5 µg/mL and incubated at room temperature for 1 hour. The cells were then washed with ice-cold PBS by centrifugation at 400 x g for 5 min three times. The cells were then stained with Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 (Invitrogen, Cat. A-11001) in ice-cold 5% goat serum/PBS at a dilution of 1:500 and incubated at room temperature in the dark for 1 hour. Following incubation, the cells were again washed three times in PBS and resuspended in ice-cold 5% goat serum/PBS. HER2 expression was then immediately analyzed using the CytoFLEX flow cytometer (Beckman Coulter Life Sciences). Unstained cells were used as a negative control.
Cell plating
For iCell CMs, CytoView MEA 24-well plates were spotted with a 5 µL droplet of fibronectin (50 µg/ mL in PBS) (Roche, Cat. 11051407001) over the microelectrode array in each well, and the plates were incubated for 1 hour at 37°C and 5% CO2. During incubation, iCell CMs were thawed according to supplier recommendations. Next, the cell suspension was transferred to a 15 mL conical tube and centrifuged at 180 x g for 5 minutes. The supernatant was aspirated, being careful not to disturb the cell pellet. Cell density and viability were determined using a hemocytometer. Cells were resuspended in iCell Cardiomyocyte Plating Medium and diluted in appropriate media to the desired working concentration of 10,000,000 CMs/ mL. The 24-well CytoView MEA plates were then removed from the incubator, and the fibronectin was aspirated. CMs were then spotted over the array in a 5 µL droplet for each well and the plate was incubated at 37°C and 5% CO2 for 1 hour. Finally, 500 µL of Plating Medium was added to each well. Integrated humidity reservoirs on the CytoView MEA plate were filled with sterile water to maintain humidity. After 2 days, a full media change and swap to Maintenance Medium was performed. Every 2-3 days thereafter during culture, 50% of the Maintenance Medium media was replaced.
For iCell glutaneurons, CytoView MEA 48-plates were treated with 50 µL of 0.1% Polyethylenimine Solution (PEI) (Sigma-Aldrich, P3143) over the microelectrode array in each well. After 1 hour incubation at 37°C and 5% CO2, each well was washed with diH20 four times to remove any excess PEI and allowed to dry overnight in the biosafety cabinet. Then, iCell glutaneurons were thawed according to supplier recommendations. Next, the cell suspension was transferred to a 15 mL conical tube and centrifuged at 180 x g for 5 minutes. The supernatant was aspirated, being careful not to disturb the cell pellet. Cell density and viability were determined using a hemocytometer. Cells were resuspended and diluted in complete (with 20 µg/mL laminin) media to the desired working concentration of 12,000,000 neurons/mL. Cells were then spotted over the array in a 10 µL droplet for each well and the plate was incubated at 37°C and 5% CO2 for 1 hour. Finally, 300 µL of additional media was added to each well of the 48-well plate. Integrated humidity reservoirs on the plate were filled with sterile water to maintain humidity. A total of 50% of the media was replaced on days 1 and 2 post-plating, and then every 2 days thereafter.
HER2-CAR T cell dosing
HER2-CAR T cells were thawed according to the manufacturer’s instructions, transferred to a 50 mL conical tube, and centrifuged at 300 x g for 5 minutes. The supernatant was aspirated, being careful not to disturb the cell pellet. Cell density and viability were determined using a hemocytometer. Cells were then diluted in complete media to the correct cell density for the desired effector:target (E:T) ratio. A total of 100 µL of the CAR T cell suspension was added to CytoView MEA 24-well plates with CMs and to CytoView MEA 48-well plates with neurons. Control wells were dosed with the same volume of only CAR T cell media. For experiments using non-transduced T cells, Pan T cells were thawed according to the manufacturer’s recommendations, counted, and dosed using the same process described above for the HER2-CAR T cells.
Viability and electrophysiology activity recording with the Maestro MEA platform
In AxIS Navigator, the Cardiac Real-Time Field Potential + Viability configuration was used for cardiac recordings and the Neural Real-Time Spontaneous + Viability configuration was used for neural recordings. These configurations acquired voltage data to assess electrophysiological function, and resistance data, via the MEA Viability module, to assess immune cell-mediated killing. Prior to recording, plates were pulled from the incubator, placed in the Maestro Pro, and allowed to equilibrate for 5 minutes. After recording, plates were returned to the incubator. Recordings were taken at 1 hour and day in vitro (DIV) 1-7 post-effector cell dose. The data from cardiac and neural recordings were analyzed using the Cardiac Analysis Tool and Neural Metric Tool, respectively.
Results
HER2-targeted CAR T cells kill iPSC-CMs in vitro
The receptor tyrosine kinase HER2 is expressed in several tissues throughout the body including the breast, skin, respiratory tract, and heart (Furrer et al., 2018). Therefore, therapies targeting HER2- expressing cancers can also damage these normal, healthy HER2-expressing tissues. We confirmed the expression of HER2 in iPSC-CMs using flow cytometry (Figure 1A) and then sought to create a model of CAR T cell on-target, off-tumor effects in vitro. To create this model, we treated iPSC-CMs with HER2-CAR T cells at effector-to-target (E:T) ratios ranging from 1:80 to 1:1 and measured the iPSC-CMs’ attachment via impedance. Imaging showed that at 7 days post-dose, CAR T cells formed clusters, indicating significant cell activation after culture with the iPSC-CMs (Figure 1B). MEA Viability (resistance) measurements from the iPSC-CMs decreased in a CAR T cell dose-dependent manner from baseline over 7 days. In the highest dose (1:1), significant decreases in resistance were seen as early as one day post-dose. At 7-days post dose, all treatment groups had lower resistance than the untreated control, including the lowest dose (1:80). These results highlight the sensitivity of the Maestro Pro and provide a proof-of-concept in vitro model of on-target, off-tumor CAR T cell killing.
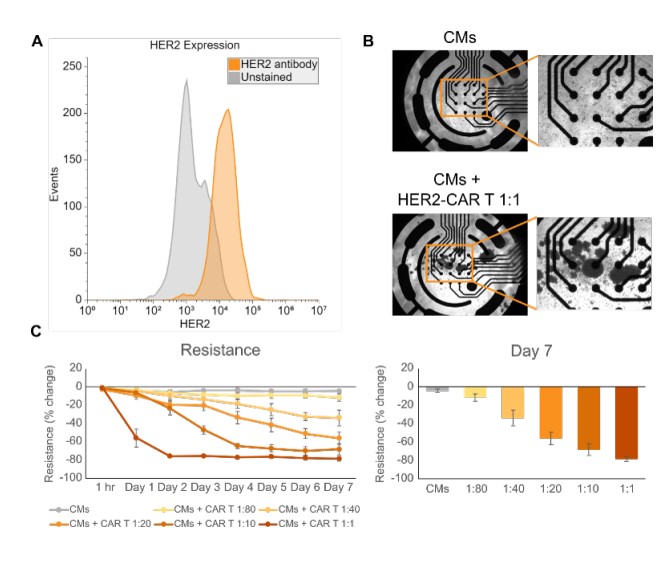
HER2-CAR T cell killing of iPSC-CMs is antigendependent
While we showed that HER2-CAR T cells killed iPSCCMs, we wanted to confirm that this cytotoxicity was due to the CAR T cells targeting the HER2 antigen on iPSC-CMs rather than general, non-specific T cell killing. Therefore, we treated iPSC-CMs with either HER2-CAR T cells or non-transduced control T cells. As expected, CMs dosed with HER2-CAR T cells at 1:2 and 1:1 ratios led to significant decreases in iPSC-CM resistance, while dosing with nontransduced T cells led to a much smaller decrease in resistance (Figure 2A). We further tested for antigen-specific killing of HER2-CAR T cells by dosing iPSC-derived neurons which do not express HER2. While HER2-CAR T cells at ratios ranging from 1:20 to 1:1 again killed iPSC-CMs, there was no significant difference between the changes in resistance of untreated neurons and those treated with the highest CAR T dose (1:1) (Figure 2B). These results confirm the specificity and antigen dependent nature of iPSC-CM cytotoxicity mediated by HER2-CAR T cells.
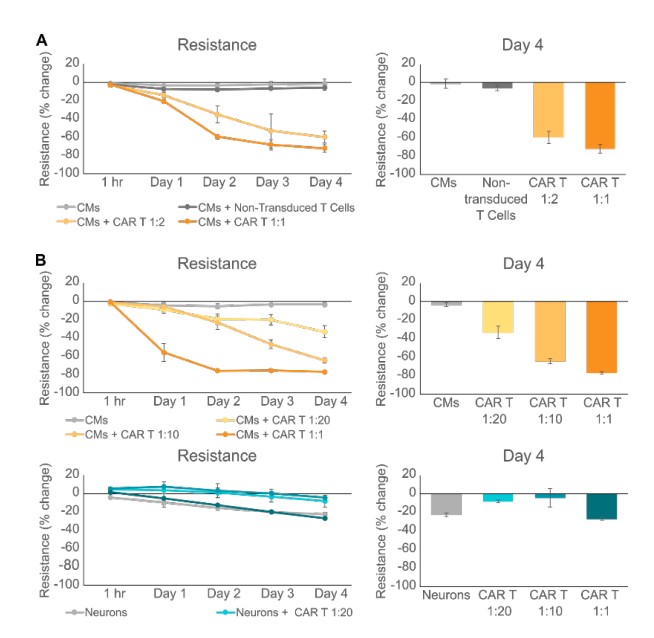
Dosing with HER2-CAR T cells induces electrophysiological changes in iPSC-CMsn
The Maestro Pro was also used to evaluate the electrophysiological function of CMs at doses and timepoints prior to cell death. We again dosed iPSCCMs with E:T ratios of HER2-CAR T cells ranging from 1:80 to 1:1 and monitored electrophysiological outputs including the Fridericia-corrected field potential duration (FPDc), beat period, and spike amplitude (Millard et al., 2018). (Figure 3A) shows representative waveforms recorded from untreated iPSC-CMs and treatment groups at baseline (predose) and at Day 3 (post-dose). CAR T dosing generally induced a leftward shift in repolarization, indicating a shorter FPD, and caused the CMs to beat faster. No Day 3 waveform is shown for the highest dose (1:1) of HER2-CAR T cells because there was complete cessation of beating after Day 1 due to cytotoxicity. (Figure 3B) shows longitudinal data for FPDc, beat period, and spike amplitude measured from each group, relative to the no treatment control. Similarly, to the 1:1 CAR T dose group, the 1:10 CAR T group lost electrophysiological activity after Day 3. FPDc and spike amplitude gradually decreased over time in a CAR T dose-dependent manner. The same is true of beat period, although a recovery towards baseline levels begins to occur at around Day 4. Interestingly, large decreases in all three metrics were apparent at 1 hour post-dose in the 1:1 CAR T dose group, illustrating that these electrophysiological changes may signal on-target, off-tumor effects even earlier than cytotoxicity data. In total, these data show that electrophysiological changes in iPSC-CMs due to HER2-CAR T cells can be detected using the Maestro Pro, and that these alterations can precede cytotoxicity.
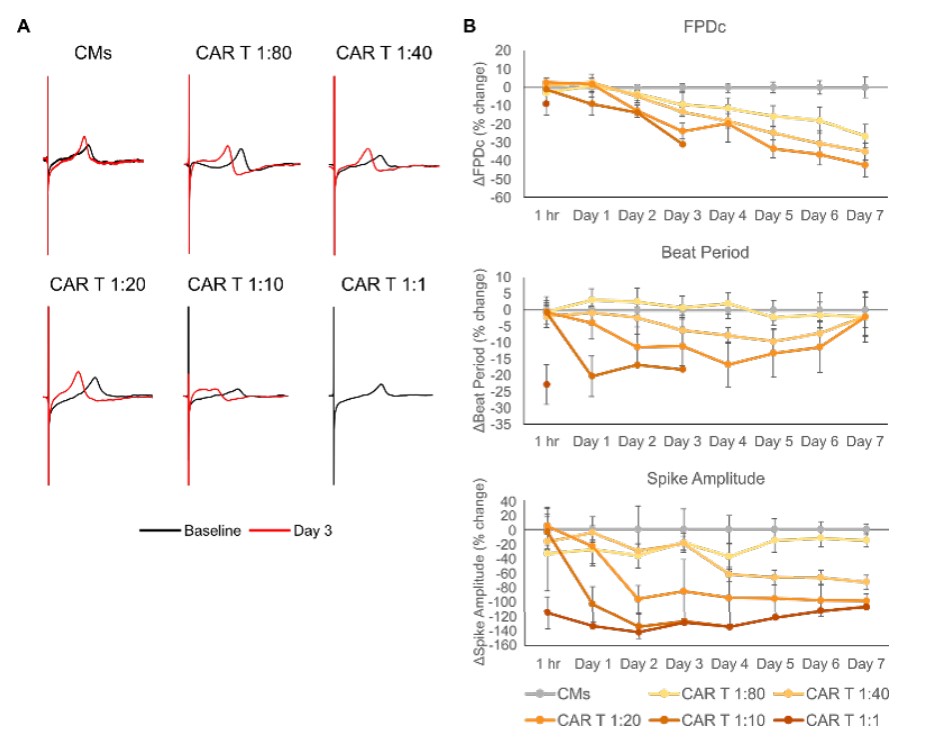
Conclusion
While CAR T cells show great potential in treating cancer, they are hampered by harmful side effects such as damage caused to non-cancerous targets expressing the CAR’s antigen. Therefore, it is important to develop in vitro models to evaluate the impact of these on-target, off-tumor effects. Here, we developed one such model composed of iPSC-CMs and HER2-CAR T cells and measured both cytotoxicity and electrophysiological data from our model using the Maestro Pro. We showed dose- and antigen-dependent killing of iPSC-CMs by HER2-CAR T cells and showed that changes in iPSC-CM electrophysiology happen in parallel to, and sometimes even prior to, viability changes. The framework established here can be applied to different or more complex models of on-target, off-tumor side effects of immunotherapies. Further work can also be done to elucidate the underlying mechanisms leading to the electrophysiological changes in iPSCCMs imparted by HER2-CAR T cell dosing. In summary, the Maestro Pro can be used to measure cytotoxicity and electrophysiological changes in models of CAR T cell on-target, off-tumor effects.
References
[1.] Aghajanian, H., Rurik, J.G., and Epstein, J.A. (2022). CAR-based therapies: opportunities for immuno-medicine beyond cancer. Nat. Metab. 4, 163–169. https://doi.org/10.1038/s42255-022-00537-5.
[2.] Flugel, C.L., Majzner, R.G., Krenciute, G., Dotti, G., Riddell, S.R., Wagner, D.L., and Abou-el-Enein, M. (2023). Overcoming ontarget, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat. Rev. Clin. Oncol. 20, 49–62. https://doi.org/10.1038/ s41571-022-00704-3.\
[3.] Frey, N., and Porter, D. (2019). Cytokine Release Syndrome with Chimeric Antigen Receptor T Cell Therapy. Biol. Blood Marrow Transplant. 25, e123–e127. https://doi.org/10.1016/j.bbmt.2018.12.756.
[4.] Furrer, D., Paquet, C., Jacob, S., Diorio, C., Furrer, D., Paquet, C., Jacob, S., and Diorio, C. (2018). The Human Epidermal Growth Factor Receptor 2 (HER2) as a Prognostic and Predictive Biomarker: Molecular Insights into HER2 Activation and Diagnostic Implications (IntechOpen).
[5.] Kitani, T., Ong, S.-G., Lam, C.K., Rhee, J.-W., Zhang, J.Z., Oikonomopoulos, A., Ma, N., Tian, L., Lee, J., Telli, M.L., et al. (2019). Human-Induced Pluripotent Stem Cell Model of Trastuzumab-Induced Cardiac Dysfunction in Patients With Breast Cancer. Circulation 139, 2451–2465. https://doi.org/10.1161/CIRCULATIONAHA.118.037357.
[6.] Millard, D., Dang, Q., Shi, H., Zhang, X., Strock, C., Kraushaar, U., Zeng, H., Levesque, P., Lu, H.-R., Guillon, J.-M., et al. (2018). Cross-Site Reliability of Human Induced Pluripotent stem cell-derived Cardiomyocyte Based Safety Assays Using Microelectrode Arrays: Results from a Blinded CiPA Pilot Study. Toxicol. Sci. 164, 550–562. https://doi.org/10.1093/toxsci/kfy110.
[7.] Sendur, M.A.N., Aksoy, S., and Altundag, K. (2013). Cardiotoxicity of novel HER2-targeted therapies. Curr. Med. Res. Opin. 29, 1015–1024. https://doi.org/10.1185/03007995.2013.807232.
[8.] Totzeck, M., Michel, L., Lin, Y., Herrmann, J., and Rassaf, T. (2022). Cardiotoxicity from chimeric antigen receptor-T cell therapy for advanced malignancies. Eur. Heart J. 43, 1928–1940. https://doi.org/10.1093/eurheartj/ehac106.
[9.] Vormittag, P., Gunn, R., Ghorashian, S., and Veraitch, F.S. (2018). A guide to manufacturing CAR T cell therapies. Curr. Opin. Biotechnol. 53, 164–181. https://doi.org/10.1016/j.copbio.2018.01.025.
[10.] Xiao, X., Huang, S., Chen, S., Wang, Y., Sun, Q., Xu, X., and Li, Y. (2021). Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J. Exp. Clin. Cancer Res. 40, 367. https://doi. org/10.1186/s13046-021-02148-6.
Authors
Benjamin Streeter, Denise Sullivan, Daniel Millard
Axion BioSystems, Atlanta, GA


