In collaboration with Miltenyi Biotec, Inc..
Key Findings
>> Maestro Z platform meets the criteria for repeatability and precision for potency testing when using a tethered liquid tumor cell line.
>> Antibody-mediated tethering of target cells is a reproducible means to quantify CAR T efficacy.
>> Miltenyi Biotec CDMO services (Miltenyi Biotec, Inc.), provide quality assay validation and execution to manufacture cell therapy products.
Background
Immunotherapy has emerged as a promising avenue of treatment for cancer, particularly for bloodborne malignancies such as acute lymphoblastic leukemia, but in vitro assays of liquid tumor cell lines can be difficult to accurately quantify. As the field of CAR T therapy continues to grow, robust and reliable potency tests remain crucial to ensuring the integrity and efficacy of treatments. Here, we demonstrate the intra-assay and interassay precision of real-time immune cell-mediated killing of nonadherent cancer cell lines (Raji) using the Maestro Z platform.
Introduction
Cell-based immunotherapies, such as CAR T cells, are emerging as a promising approach to therapeutic intervention against cancer. Developing cell and gene therapy (CGT) products requires reliable and reproducible methods of quantifying critical quality attributes, such as potency, to ensure product strength and consistency. A variety of assays can be used to assess potency; however, these tests must be qualified for assay performance to ensure both intra-assay and interassay precision. Although inherently variable, biological assays are often the best methods to investigate a product’s mechanism of action and predict clinical outcomes.
Previous work has shown that impedance-based potency assays provide a non-invasive, real-time measure of effector cell-mediated cytotoxicity, thus reducing cell manipulation that impacts data reliability. Here, we describe an in vitro potency assay demonstrating low variability and high precision across replicates, analysts, and days. Effector cell-mediated cytotoxicity was performed using a CD19- positive liquid tumor cell line (Raji) tethered to the well surface using an anti-CD40 antibody.
The CD19-specific CAR T effector cells were added 24 hours after the target cells and co-cultured with the target cells across a range of effector to target (E:T) ratios and cytolysis was monitored for 3 days. Real-time measurement of cell-mediated cytotoxicity also allowed for the calculation of kill time 50 (KT50), defined as the time required for 50% cytolysis of the target cells. Repeatability was calculated across 3 replicates per E:T ratio. The co-culture experiment was performed in duplicate by a second analyst and on 2 subsequent days for a comparison of 3 plates and 2 operators to evaluate interassay precision. In addition to this, CAR T-mediated cell killing in a multiplate impedance system was also investigated for plate-to-plate variability. The percent coefficient of variation (% CV), a statistical measure that describes the precision and repeatability of an assay, was calculated to assess variability between assay replicates, operators, and plate replicates.
In all conditions tested, the percent CV of cytolysis for all E:T ratios was below 20% (after normalization) for both repeatability (n=3) and intermediate precision (n=3) illustrating the repeatability and precision of the assay. These data support using an impedance-based potency assay for evaluating and characterizing immunotherapy products.
Materials and Methods
Cells and reagents
Frozen Raji-luciferase tumor cells maintained at Miltenyi Biotec (San Jose, CA) were thawed and expanded in RMPI1640 (Gibco cat. 11875-093) containing 10% FBS (Gibco cat. A38402-01) at 37 °C with 5% CO2. At 1 to 3 days prior to the assay, Raji-luciferase cells were split into 2 T75 flasks for 2 operators. Each operator harvested 1 T75 flask and independently prepared Raji-luciferase cells for the assay.
A total of 2 vials of frozen CAR T cells (Miltenyi Biotec, San Jose, CA) were thawed separately in RPMI1640/10% FBS by 2 operators. Each operator prepared 1 vial of CAR T cells for the assay, independently and immediately after thawing.
Maestro Z assay platform
The Maestro Z platform (Axion BioSystems) uses impedance measurements (ohms, Ω) to quantify the presence of cells on electrodes embedded in the bottom of the wells of CytoView-Z plates (Axion BioSystems). Cellular impedance is a well-established technique for measuring cell attachment, spreading, proliferation, coupling, membrane integrity (cell death), and subtle changes in cell conformation. Detection is noninvasive and label free, so it can quantify dynamic cellular responses over minutes, hours, and days. The Maestro Z built-in environmental chamber finely controls temperature and CO2, ensuring a consistent, optimal experimental environment.
Validation method
The attributes of repeatability and intermediate precision were assessed in this validation study. Refer to the section “CAR T cell-mediated killing assay,” below for the execution protocol.
Repeatability expresses the precision under the same operating condition over a short interval of time. In this study, 4 CAR T cell concentrations were tested at E:T ratios 10:1, 5:1, 5:2, and 5:4 in 3 replicates per condition and per operator. The mean value, standard deviation, and % CV were calculated from the replicates within the same conditions for the % cytolysis and KT50. The % CVs were reported for the assessment of repeatability.
Intermediate precision expresses the intra-laboratory variations caused by the potential sources of variability, such as different days, analysts, equipment, and environmental conditions. In this study, the assay setup is the same as that described in the repeatability above, but the precision was evaluated between 2 operators and among 3 different CytoView-Z 96-well plates. A total of 3 identical assay runs were executed during 3 different time periods.
For each run, 1 CD40-coated CytoView-Z plate was shared by 2 operators. The target cells and CAR T cells were prepared separately by 2 operators at a coordinated time. The cells prepared by 1 operator were loaded to the first half of the plate, and the cells prepared by the other operator were loaded to the second half of the plate. The mean value, standard deviation, and CV were calculated between 2 operators and among 3 different CytoView-Z 96-well plates for both % cytolysis and KT50. The % CV between operators and % CV among the plates were reported for evaluating intermediate precision.
CAR T cell-mediated killing assay
The CD40 antibody ( # 130-094-133 ) tethering solution from Miltenyi Biotec was prepared in PBS. CytoView-Z 96-well plate (Axion BioSystems) were coated with 50 μL of antibody solution (4 μg/mL) per well and incubated at 4 °C overnight. After incubation, excess surface coating was aspirated from each well. Then, 50 μL of pre-warmed RPMI1640/10% FBS were added to each well. The plate was docked on the Maestro Z platform to record a media reference.
Raji-luciferase target cells were collected from 1 T75 flask, counted, and prepared separately by each operator at a concentration of 2.5e5 viable cells/mL. Each operator added 100 µL Raji-luciferase cells (2.5e4 cells/well) to the designated wells and 100 µL RPMI1640/10% FBS to the designated media control and effector-only control wells.
The plate was allowed to rest at room temperature for 1 hour before docking on the Maestro Z, which incubated the plate at 37 °C and 5% CO2. Integrated humidity reservoirs on the CytoView-Z 96 were filled with sterile water to maintain humidity. Raji-luciferase cell tethering was monitored in the Maestro Z for 24±4 hours.
For each operator, 1 vial of CAR T cells were thawed in RPMI1640/10% FBS and viable cells were counted using the NucleoCounter® NC-200™ automated cell counter (ChemoMetec). The CAR T cell concentration was calculated based on viable cell concentration and transduction efficiency (% CAR T cells in viable cell population). The starting CAR T cell-working solution was prepared at 2.5e6 CAR T cells per mL in RPMI1640/10% FBS for each E:T ratio. Two-fold serial dilutions were prepared at 1.25e6 CAR T/mL, 6.25e5 CAR T/mL and 3.13e5 CAR T/mL from the starting CAR T cell-working solution.
The CytoView-Z 96-well plate was removed from the Maestro Z and placed in a biosafety cabinet. Then, 100 µL per well of CAR T cells at 2.5e6 CAR T/mL to 3.13e5 CAR T/mL were added to the designated wells of the CytoView-Z 96. The resulting E:T ratios were 10:1, 5:1, 5:2, and 5:4, respectively. The same volume of media was added to the “no treatment control” and “media only control” groups. The plate was then allowed to rest for 30 minutes at room temperature before again docking onto the Maestro Z. Target cell proliferation and death was then monitored over time on the Maestro Z.
Data analysis
Cytolysis was analyzed at 24- and 48-hours post-CAR T cell addition. Time reference with normalization was set in AxIS Z software at the time of the CAR T cell addition.
All data and graphs were exported from AxIS Z for all additional computations. Outlier removal was performed by identification and removal of data points that were greater than 3 standard deviations from the mean within a treatment group. The % CV for each treatment condition was calculated as:
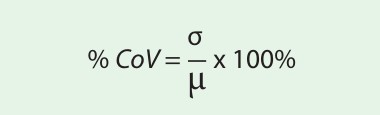
where µ and σ represent the mean and standard deviation. The calculated % CVs should be 20% or less to demonstrate minimal variability.
Endpoint calculations
Treatment group resistance measurements were corrected for “treatment-only control,” values (CAR T cells alone) using the AxIS Z software linked controls feature.
The % cytolysis was used to quantify cell death and was calculated using the following equation:
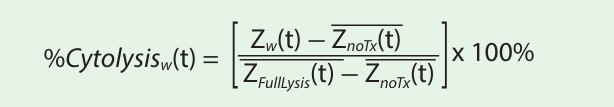
where:

Real-time measurement of cell-mediated cytotoxicity allowed the calculation of KT50, defined as the time required for 50% cytolysis of the target cells. The % cytolysis and KT50 were calculated and exported using the AxIS Z software.
Results
Repeatability
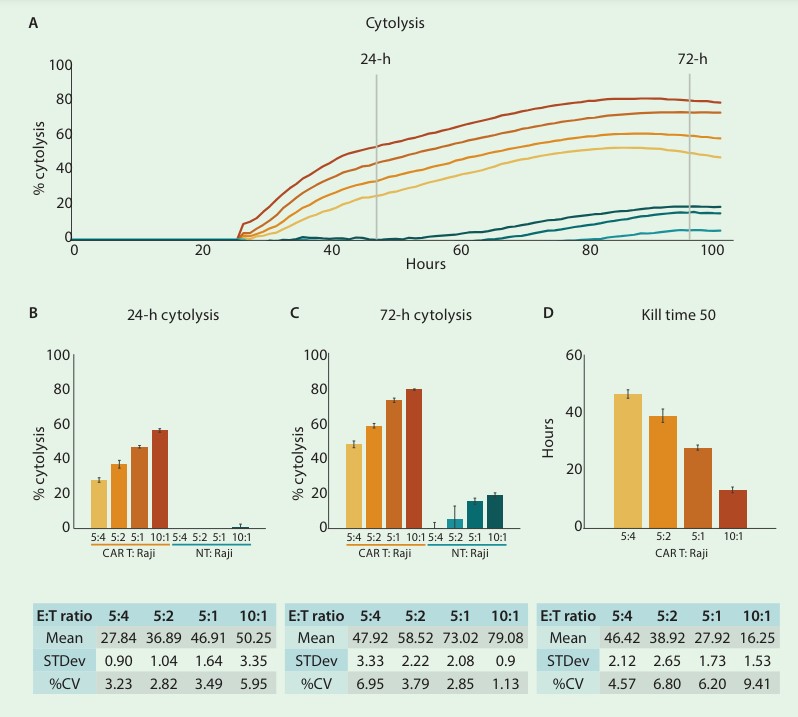
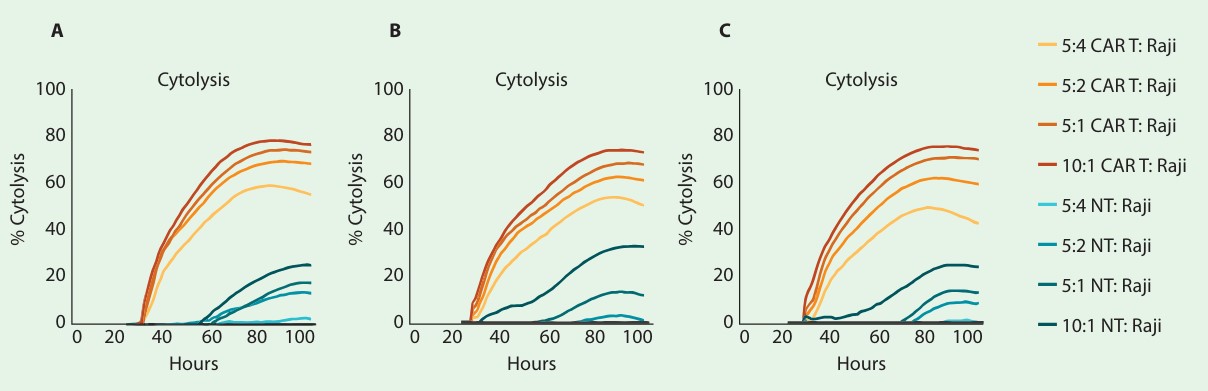
Development and validation of potency assays is a crucial step in the immunotherapy pipeline. In order to qualify a potency bioassay, researchers must report the intra-assay precision or repeatability. To assess repeatability, the cytolysis of CD19-expressing Raji cells by CD19-specific CAR T cells was measured across replicates at 4 different E:T ratios (10:1, 5:1, 5:2, and 5:4). Figures 1 A-C show time series and endpoint (24- and 72-hours post-CAR T addition) % cytolysis of target cells treated with either CAR T or non-transduced T cells.
As expected, the number of CAR T cells highly correlated with the percent cytolysis of the target cells, with the highest E:T ratio reaching ~80% cytolysis. This was further confirmed by comparing KT50 values for each E:T ratio (fig. 1D). The mean and standard deviation, as well as the standard deviation of the % CV, were calculated for both endpoint cytolysis and KT50. The % CV across all replicates was below 10% for endpoint cytolysis and KT50, indicating high repeatability of assay replicates.
Intermediate precision – inter-operator
Intermediate precision assesses the consistency of results obtained from the same method, instruments, and conditions within the same laboratory but on different occasions or by different operators. Intermediate precision helps evaluate the robustness and reliability of analytical methods by considering the inherent variability that can occur due to factors such as equipment calibration and human influences.
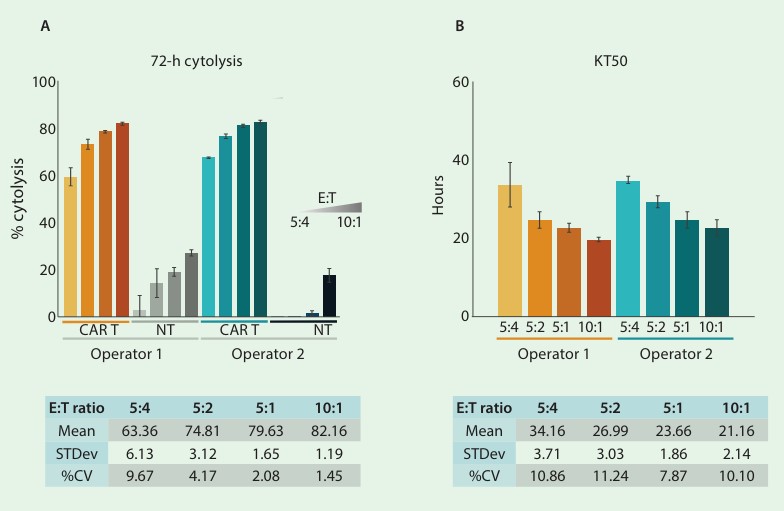
In this study, intermediate precision results were generated by 2 analysts who prepared replicate sample preparations with their own cell vials and reagents. Each analyst seeded Raji target cells to anti-CD40 coated plates at a density of 25K cells. After 24 hours, the analysts added CD-19 CAR T cells or nontransduced (NT) T cells at 4 different E:T ratios (10:1, 5:1, 5:2, and 5:4). Endpoint cytolysis and KT50 values were compared between operators and overall mean, standard deviation, and % CV were determined. The % cytolysis at 72 hours following CAR T treatment was 63.36%, 74.81%, 79.63%, and 82.16% for each increasing E:T ratio, respectively (fig. 2A).
The KT50 values inversely correlated with the number of effector cells, such that as the number of effector cells increased, the time required for 50% cytolysis of the target cells decreased (fig. 2B). The % CV of less than 15% for all conditions demonstrates the high consistency and low analyst-to-analyst variability of the impedance assay.
Intermediate precision – inter-assay
The aforementioned setup was repeated on 3 separate days and plates to evaluate interassay precision. Figure 3A-C shows the time series of each plate/day. Endpoint cytolysis and KT50 for each plate are displayed in figure 4A and 4B, respectively. The overall mean, standard deviation, and % CV are shown in the tables. Endpoint cytolysis values show high plate-to-plate consistency across all E:T ratios with % CV less than 15%.
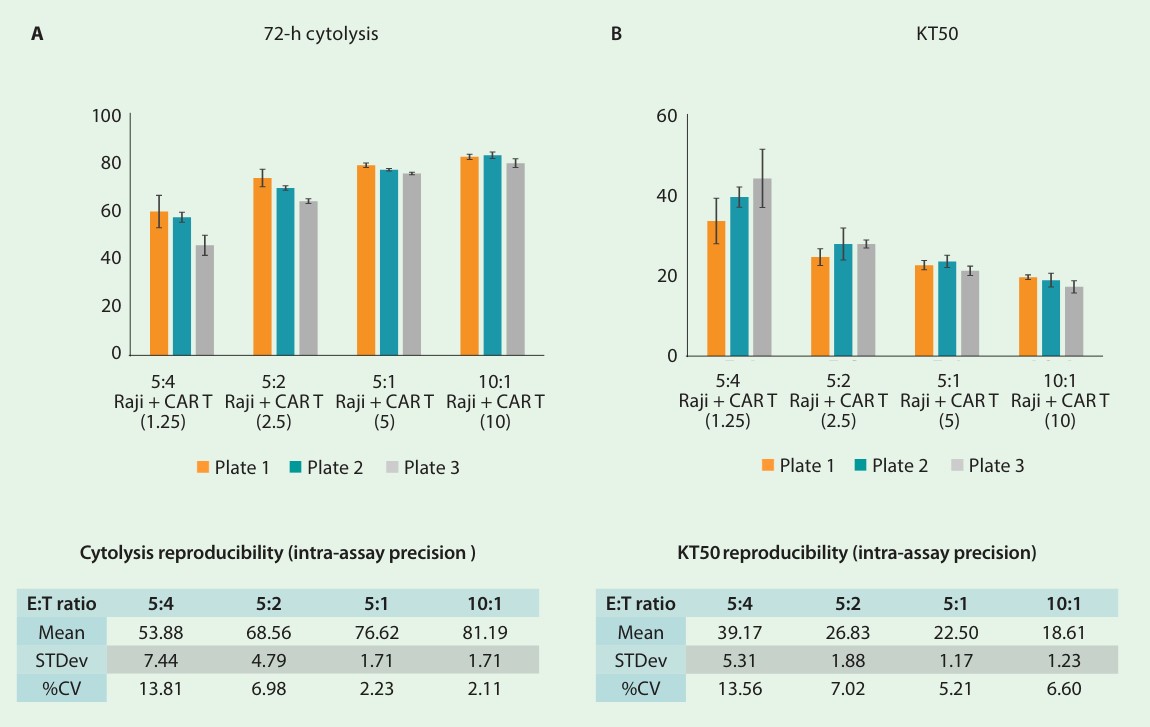
The KT50 values were used to compare overall cytolysis between plates and exhibited a low % CV of around 15% or less. These data taken together indicate that the Maestro Z platform meets the criteria for precision for potency testing when using a tethered liquid tumor cell line.
Conclusion
Potency test validation is critical to ensure cell-based therapies’ efficacy, safety, and reproducibility. Validation procedures confirm the therapeutic potential, ensure that regulatory approval processes are followed, and facilitate improved patient outcomes. Here, we demonstrate a qualified method for in vitro immune cell-mediated killing assays with non-adherent target cell types. Low variability between replicates, operators, and plates of CAR T killing reflects the robustness of the Maestro Z assay, as well as the proficiency and expertise of CDMO analysts. In summary, the potency of CAR T cell killing of liquid tumor cell lines can be quantified using the Maestro Z, making it a valuable tool for CAR T cell discovery and manufacturing.
References
1. U.S. Department of Health and Human Services Food and Drug Administration, Center for Biologics Evaluation and Research. (2011) Potency Tests for Cellular and Gene Therapy Products. FDA-2008-D-0520.
Authors
Danielle Califano1, Jianjie Jiang2, Dirk Windgassen2, Caitlyn De La Flor2, Stacie Chvatal1, Denise Sullivan1, and Daniel Millard1
1Axion BioSystems, Atlanta, GA, USA
2Miltenyi Biotec, Inc., San Jose, CA USA