Safety Pharmacology Society, 2019
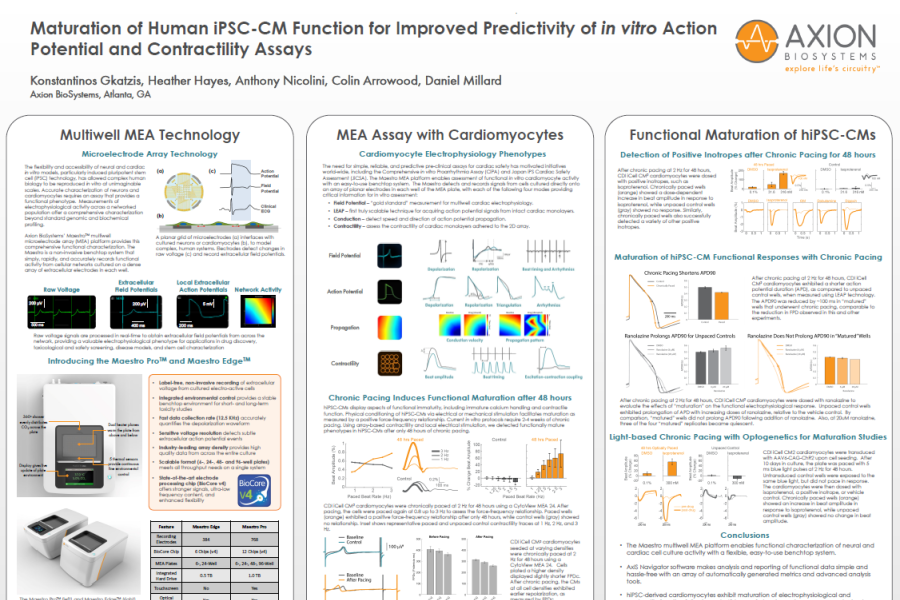
Multiwell MEA Technology
Microelectrode array technology
The flexibility and accessibility of neural and cardiac in vitro models, particularly induced pluripotent stem cell (iPSC) technology, has allowed complex human biology to be reproduced in vitro at unimaginable scales. Accurate characterization of neurons and cardiomyocytes requires an assay that provides a functional phenotype. Measurements of electrophysiological activity across a networked population offer a comprehensive characterization beyond standard genomic and biochemical profiling. Axion BioSystems’ Maestro™ multiwell microelectrode array (MEA) platform provides this comprehensive functional characterization. The Maestro is a non-invasive benchtop system that simply, rapidly, and accurately records functional activity from cellular networks cultured on a dense array of extracellular electrodes in each well.
MEA Assay with Cardiomyocytes
Cardiomyocyte Electrophysiology Phenotypes
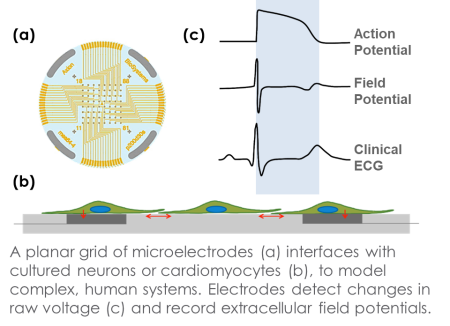
The need for simple, reliable, and predictive pre-clinical assays for cardiac safety has motivated initiatives world-wide, including the Comprehensive in vitro Proarrhythmia Assay (CiPA) and Japan iPS Cardiac Safety Assessment (JiCSA). The Maestro MEA platform enables assessment of functional in vitro cardiomyocyte activity with an easy-to-use benchtop system. The Maestro detects and records signals from cells cultured directly onto an array of planar electrodes in each well of the MEA plate, with each of the following four modes providing critical information for in vitro assessment:
- Field Potential – “gold standard” measurement for multiwell cardiac electrophysiology.
- LEAP – first truly scalable technique for acquiring action potential signals from intact cardiac monolayers.
- Conduction – detect speed and direction of action potential propagation.
- Contractility – assess the contractility of cardiac monolayers adhered to the 2D array
Chronic Pacing Induces Functional Cardiomyocyte Maturation after 48 hours
hiPSC-CMs display aspects of functional immaturity, including immature calcium handling and contractile function. Physical conditioning of hiPSC-CMs via electrical or mechanical stimulation facilitates maturation as measured by a positive force-frequency relationship. Current in vitro protocols require 2-4 weeks of chronic pacing. Using array-based contractility and local electrical stimulation, we detected functionally mature.
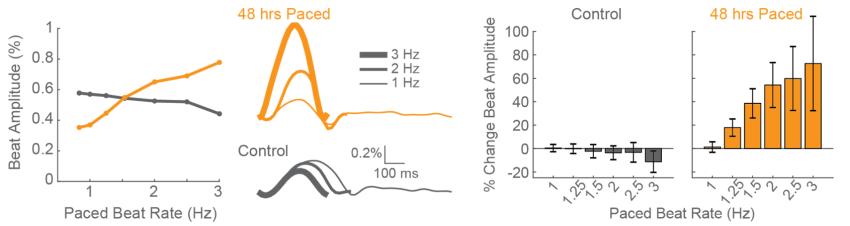
CDI iCell CM2 cardiomyocytes were chronically paced at 2 Hz for 48 hours using a CytoView MEA 24-well plate. After pacing, the cells were paced again at 0.8 up to 3 Hz to assess the force-frequency relationship. Paced wells (orange) exhibited a positive force-frequency relationship after only 48 hours, while control wells (gray) showed no relationship. Inset shows representative paced and unpaced control contractility traces at 1 Hz, 2 Hz, and 3 Hz.
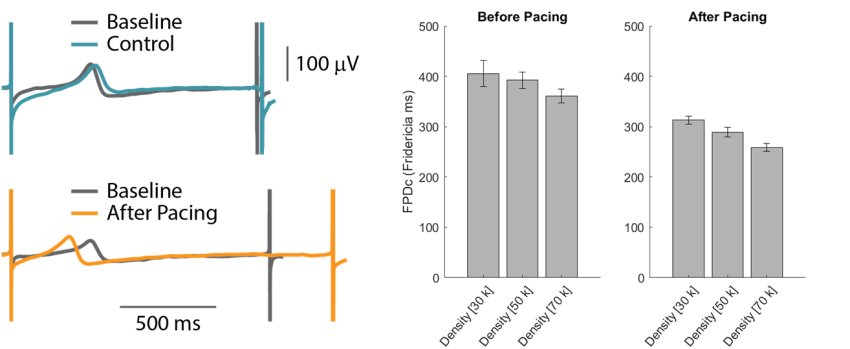
CDI iCell CM2 cardiomyocytes seeded at varying densities were chronically paced at 2 Hz for 48 hours using a CytoView MEA 24-well plate. Cells plated a higher density displayed slightly shorter FPDc. After chronic pacing, the CMs at all cell densities exhibited earlier repolarization, as measured by FPDc.
Functional Maturation of hiPSC-CMs
Detection of Positive Inotropes after Chronic Pacing for 48 hours
After chronic pacing at 2 Hz for 48 hours, CDI iCell CM2 cardiomyocytes were dosed with positive inotropes, such as isoproterenol. Chronically paced wells (orange) showed a dose-dependent increase in beat amplitude in response to isoproterenol, while unpaced control wells (gray) showed no response. Similarly, chronically paced wells also successfully detected a variety of other positive inotropes.
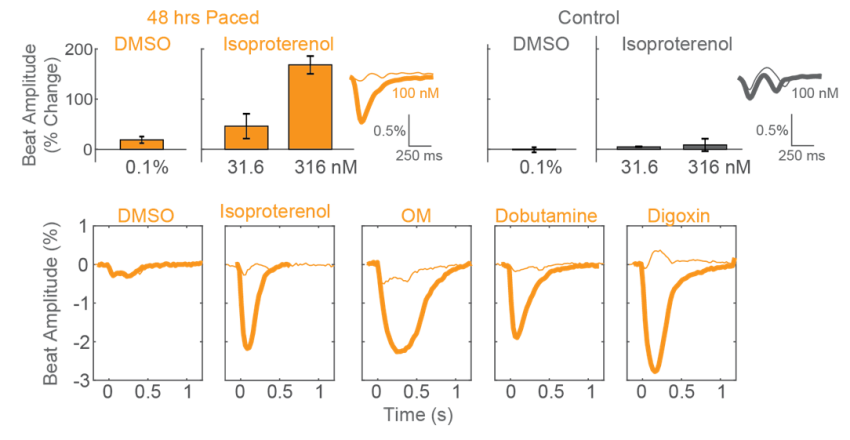
Maturation of hiPSC-CM Functional Responses with Chronic Pacing
After chronic pacing at 2 Hz for 48 hours, CDI iCell CM2 cardiomyocytes exhibited a shorter action potential duration (APD), as compared to unpaced control wells, when measured using LEAP technology. The APD90 was reduced by ~100 ms in “matured” wells that underwent chronic pacing, comparable to the reduction in FPD observed in this and other experiments.
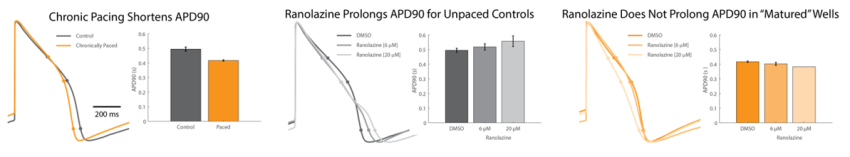
After chronic pacing at 2 Hz for 48 hours, CDI iCell CM2 cardiomyocytes were dosed with ranolazine to evaluate the effects of “maturation” on the functional electrophysiological response. Unpaced control wells exhibited prolongation of APD with increasing doses of ranolazine, relative to the vehicle control. By comparison, “matured” wells did not prolong APD90 following addition of ranolazine. Also, at 20uM ranolazine, three of the four “matured” replicates became quiescent.
Light-based Chronic Pacing with Optogenetics for Maturation Studies
CDI iCell CM2 cardiomyocytes were transduced with AAV6-CAG-ChR2 upon cell seeding. After 10 days in culture, the plate was paced with 5 ms blue light pulses at 2 Hz for 48 hours. Untransduced control wells were exposed to the same blue light, but did not pace in response. The cardiomyocytes were then dosed with isoproterenol, a positive inotrope, or vehicle control. Chronically paced wells (orange) showed an increase in beat amplitude in response to isoproterenol, while unpaced control wells (gray) showed no change in beat amplitude.
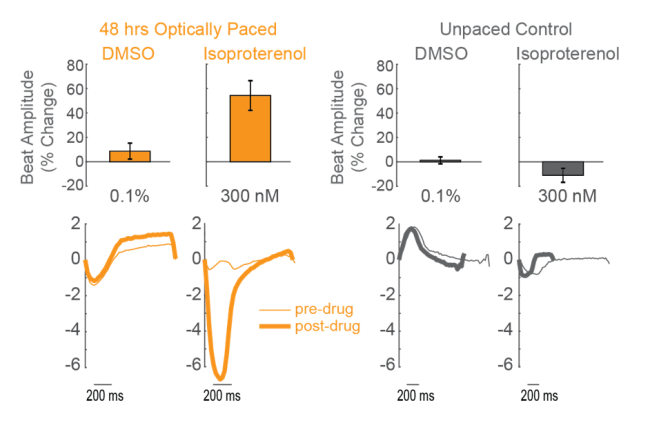
Conclusions
- The Maestro in vitro multiwell MEA platform enables functional characterization of neural and cardiac cell culture activity with a flexible, easy-to-use benchtop system.
- AxIS Navigator software makes analysis and reporting of functional data simple and hassle-free with an array of automatically generated metrics and advanced analysis tools.
- hiPSC-derived cardiomyocytes exhibit maturation of electrophysiological and contractile function following only 48 hours of chronic pacing, as measured using turnkey assays for action potential and contractility measurements.