BrainPhys™ Neuronal Medium is a new, defined and serum‑free neuronal culture medium for the culture of both primary neurons and neurons derived from human pluripotent stem cells (hPSCs); including embryonic stem (ES) and induced pluripotent stem (iPS) cells. Traditional neuronal culture media, such as Neurobasal Medium and Dulbecco’s Modified Eagle Medium (DMEM) support cell survival, but impair neurological activities, including action potential generation and synaptic activity (Table 1). BrainPhys™ was designed by Dr. Cedric Bardy in Dr. Fred H. Gage’s laboratory to better support in vitro neuronal function. Neuronal cultures matured in BrainPhys™ Neuronal Medium experience central nervous system (CNS)‑like physiological conditions and develop a higher proportion of synaptically active neurons. Furthermore, using BrainPhys™ Neuronal Medium increases the physiological relevance of in vitro neuroscience research, thus increasing the likelihood of successfully translating scientific discoveries from the laboratory to the clinic. As a versatile neuronal basal medium, BrainPhys™ Neuronal Medium may be used with many diverse neuronal culture and differentiation protocols, in combination with appropriate neuronal supplements, cytokines or small molecules. Because BrainPhys™ Neuronal Medium supports neuronal function, assays of neuronal activity (such as calcium imaging, microelectrode array (MEA)-based recording and optogenetics) can be performed within the established cultures, without the need to replace media. This streamlines experimental workflows and minimizes sudden or frequent changes to the cultures.
| Advantages | |
|---|---|
| Physiological | More Representative of the brain's extracellular environment. |
| Active | Improved neuronal function and a higher proportion of synaptically active neurons. |
| Streamlined | Perform functional assays without replacing media. |
| Versatile | Supports long-term culture of ES/iPS cell-and CNS-derived neurons. |
| Reliable | Rigorous raw material screening and quality control ensure minimal lot-to-lot variability. |
Applications of BrainPhys™ Neuronal Medium
- Culture of primary mouse or rat neurons
- Differentiation and maturation of human ES/iPS cell‑derived neurons
- Microelectrode array-based recording of neuronal activity
- Fluorescence-based live imaging, including calcium imaging and optogenetic stimulation and recording
- Transdifferentiation (lineage conversion) of somatic cells to neurons
Primary Neuronal Culture
Primary neuronal cultures have long been a powerful system with which to study neuronal biology in a controlled environment. To obtain healthy cultures with good morphology and cellular function, the use of high-quality media and supplements is crucial. For optimal neuronal function and survival, plate mouse or rat neurons in a traditional neuronal medium (e.g. NeuroCult™ Neuronal Basal Medium, supplemented with NeuroCult™ SM1 Neuronal Supplement) and transition cultures to BrainPhys™ Neuronal Medium, supplemented with NeuroCult™ SM1, after 5 days in vitro (DIV).
After 14 and 21 DIV, cultures matured in BrainPhys™ Neuronal Medium show large numbers of viable neurons, with minimal cell clumping and extensive neurite outgrowth and branching (Figure 1A,C). Neuronal morphology is consistent with neurons cultured in a traditional neuronal medium (Figure 1B,D). The number of viable neurons after 21 DIV is consistently greater in BrainPhys™ Neuronal Medium‑matured cultures, regardless of whether NeuroCult™ Neuronal Basal Medium or Neurobasal Medium is used as the initial plating medium (Figure 2).
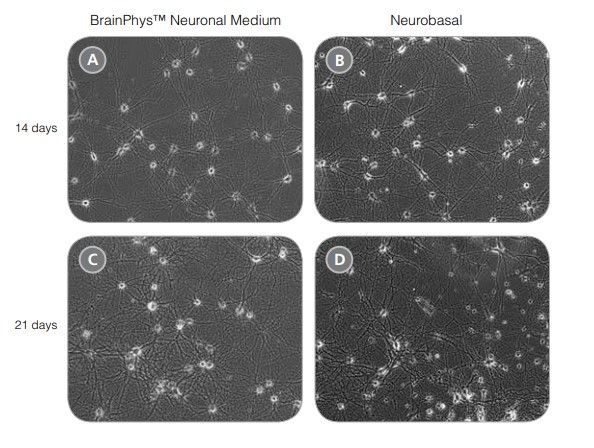
Figure 1. Rodent Neurons Matured in BrainPhys™ Neuronal Medium are Healthy and Morphologically Mature
(A,C) Primary rat E18 cortical neurons were plated in NeuroCult™ Neuronal Basal Medium, supplemented with NeuroCult™ SM1 Neuronal Supplement. After 5 DIV, the cultures were transitioned to BrainPhys™ Neuronal Medium, supplemented with NeuroCult™ SM1, by performing half-medium changes every 3 - 4 days. Neurons were cultured for 14 (A) or 21 (C) DIV. (B,D) Primary rat E18 cortical neurons were plated and matured in a traditional neuronal medium (Neurobasal Medium), supplemented with NeuroCult™ SM1 Neuronal Supplement for 14 (B) or 21 (D) DIV. Neuronal morphology of BrainPhys™ Neuronal Medium matured neurons is consistent with neurons plated and matured in a traditional neuronal medium.
Neurons cultured in BrainPhys™ Neuronal Medium are functionally mature, and show improved synaptic activity compared to those cultured in a traditional neuronal medium (Figure 3). The frequency and amplitude of spontaneous excitatory (AMPA receptor-mediated) and inhibitory (GABA receptor-mediated) synaptic currents are increased in BrainPhys™ Neuronal Medium-matured cultures. Furthermore, using a MEA system (Axion Biosystems), the mean firing rate and percentage of active electrodes of neurons cultured in BrainPhys™ Neuronal Medium increased markedly over time, whereas both remained low in neurons cultured in a traditional neuronal medium (TNM) (Figure 4). Marker analysis confirms that neurons cultured in BrainPhys™ Neuronal Medium are phenotypically mature, as indicated by the highly branched morphology and appropriate expression of MAP2 and Synapsin 1 (Figure 5).
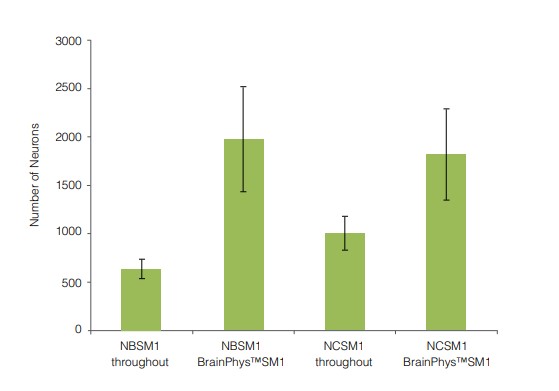
Figure 2. Primary Neuronal Cultures Matured in BrainPhys™ Neuronal Medium Have Greater Numbers of Neurons
Primary rat E18 cortical neurons were plated in either NeuroCult™ Neuronal Basal Medium (NCSM1) or Neurobasal Medium (NBSM1), supplemented with NeuroCult™ SM1. After 5 DIV, half of the cultures were transitioned to BrainPhys™ Neuronal Medium, supplemented with NeuroCult™ SM1, by performing half‑medium changes every 3 - 4 days. The other half of the cultures were maintained in the same medium as used for plating. After 21 DIV, more neurons were evident in the cultures matured in BrainPhys™ Neuronal Medium, regardless of whether NeuroCult™ Neuronal Basal Medium or Neurobasal Medium was used as the plating medium. (n = 2, mean ± SEM [triplicate wells were set up for each experiment]).
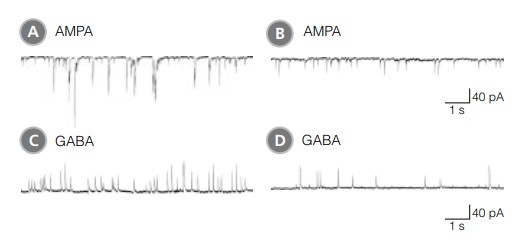
Figure 3. Rodent Neuronal Cultures Matured in BrainPhys™ Neuronal Medium Show Improved Excitatory and Inhibitory Synaptic Activity in Classic Electrophysiology Assays
(A,C) Primary rat E18 cortical neurons were plated in NeuroCult™ Neuronal Basal Medium, supplemented with NeuroCult™ SM1 Neuronal Supplement. After 5 DIV, the cultures were transitioned to BrainPhys™ Neuronal Medium, supplemented with NeuroCult™ SM1 Neuronal Supplement, by performing half-medium changes every 3 - 4 days. Neurons were cultured for 21 DIV. (B,D) Primary rat E18 cortical neurons were plated and matured in a traditional neuronal medium (Neurobasal Medium), supplemented with NeuroCult™ SM1 Neuronal Supplement for 21 DIV. (A,C) Neurons matured in BrainPhys™ Neuronal Medium showed spontaneous excitatory (AMPA‑mediated; A) and inhibitory (GABA-mediated; C) synaptic events. The frequency and amplitude of spontaneous synaptic events is consistently greater in neuronal cultures matured in BrainPhys™ Neuronal Medium, compared to neurons plated and matured in a traditional neuronal medium (B,D). Traces are representative.
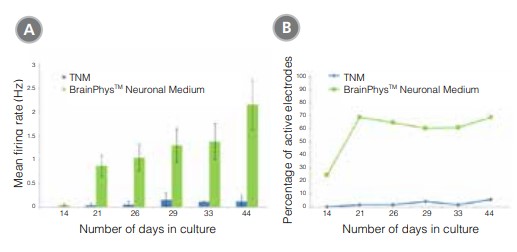
Figure 4. Primary Neuronal Cultures Matured in BrainPhys™ Neuronal Medium Show Improved Electrical Activity in Microelectrode-Array Systems
Primary rat E18 cortical neurons were plated in a traditional neuronal medium (TNM; Neurobasal Medium supplemented with NeuroCult™ SM1). After 5 DIV, half of the cultures were transitioned to BrainPhys™ Neuronal Medium, supplemented with NeuroCult™ SM1, by performing half‑medium changes every 3 - 4 days. The other half of the cultures were maintained in TNM throughout. The electrical activities of the neuronal cultures were measured twice a week using a microelectrode array (MEA) system (Axion Biosystems). (A) The mean firing rate of neurons cultured in BrainPhys™ Neuronal Medium increased over time, whereas the mean firing rate of neurons in the TNM condition remained low over time (n=1; mean ± SEM, 128 electrodes). (B) The percentage of active electrodes (>0.005 Hz) of neurons matured in BrainPhys™ Neuronal Medium increased from 24% on day 14 to 69% on day 21, and then remained stable at 60 – 70% from days 21 – 44. In contrast, < 5% of electrodes were active in the TNM condition over the same 6-week period.
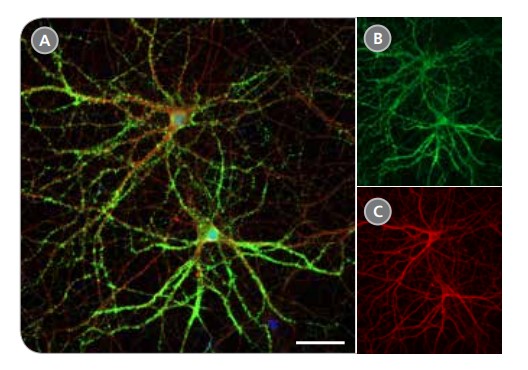
Figure 5. Expression of Pre-Synaptic Markers in Rodent Neurons Matured in BrainPhys™ Neuronal Medium
Primary rat E18 cortical neurons were plated in NeuroCult™ Neuronal Basal Medium, supplemented with NeuroCult™ SM1 Neuronal Supplement. After 5 DIV, the cultures were transitioned to BrainPhys™ Neuronal Medium, supplemented with NeuroCult™ SM1 Neuronal Supplement, by performing half-medium changes every 3 - 4 days. Neurons cultured for 21 DIV are phenotypically mature, as indicated by the presence of an extensive dendritic arbor. The pre-synaptic marker synapsin (A,B; green) is concentrated in discrete puncta distributed along the somata and dendritic processes, as defined by the dendritic marker MAP2 (A,C; red). Scale bar= 50 µm.
hPSC-Derived Neuronal Culture
hPSCs, including ES and iPS cells, are a valuable tool for researchers aiming to model human neurological development and disease. By generating iPS cells from patient samples, disease- and patient-specific neural stem cells, neurons and glia can be obtained. Published protocols are available for the generation of many different neuronal subtypes, using a basal medium together with neural supplements, such as NeuroCult™ SM1 Neuronal Supplement (based on the published B27 formulation2 ) and N2 supplement,3 and various cytokines and small molecules. Using BrainPhys™ Neuronal Medium as the basal medium for hPSC-derived neural progenitor cell (NPC) differentation and neuronal maturation will generate a more neurophysiologically active culture that better represents the human brain environment.1 BrainPhys™ Neuronal Medium may also be used for direct lineage conversion of somatic cells into neurons, without transitioning through a hPSC intermediate.1
Neurons can be generated efficiently from hPSC-derived NPCs using BrainPhys™ Neuronal Medium supplemented with NeuroCult™ SM1, N2 Supplement-A, glial cell derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), ascorbic acid and cyclic AMP (cAMP). After 14 days of differentiation in BrainPhys™ Neuronal Medium, neurons express the synaptic marker Synapsin 1 and the mature neuronal marker MAP2, consistent with neurons cultured in a traditional basal medium under the same supplementation conditions (DMEM/F12) (Figure 6A-B). After 44 days of differentiation and maturation in BrainPhys™ Neuronal Medium, a large number of viable neurons with extensive branching make up a dense neurite network that can be observed under phase-contrast microscopy (Figure 7A). In comparison, traditional cultures show increased neuronal vacuolation and cellular debris after differentiation and maturation in a traditional culture medium. (Figure 7B). Marker analysis confirms that neurons cultured in BrainPhys™ Neuronal Medium express MAP2 and Synapsin 1 after 44 days in vitro (Figure 6C). Patch clamp analysis shows that neurons cultured in BrainPhys™ Neuronal Medium are functionally mature and show improved synaptic activity, compared to those cultured in a traditional basal medium (Figure 8). The frequency and amplitude of spontaneous excitatory (AMPA receptor-mediated) and inhibitory (GABA receptor-mediated) synaptic currents are increased in BrainPhys™ Neuronal Medium-matured cultures, compared to neurons matured in a traditional medium. hPSC‑derived neurons have been successfully matured in BrainPhys™ Neuronal Medium for up to 70 days in vitro.
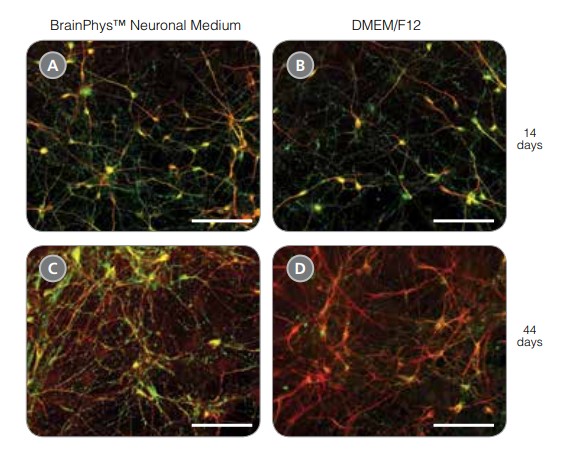
Figure 6. hPSC-Derived Neurons Generated in BrainPhys™ Neuronal Medium Express Markers of Neuronal Maturity After 14 and 44 Days of Differentiation
NPCs were generated from H9 cells using STEMdiff™ Neural Induction Medium in an embryoid body-based protocol. Next, NPCs were cultured in (A,C) BrainPhys™ Neuronal Medium, supplemented with 2% NeuroCult™ SM1 Supplement, 1% N2 Supplement-A, 20 ng/mL GDNF, 20 ng/mL BDNF, 1 mM db-cAMP and 200 nM ascorbic acid to initiate neuronal differentiation, or (B,D) DMEM/F12 under the same supplementation conditions. After 14 and 44 days of differentiation and maturation, neurons express the synaptic marker Synapsin 1 (green) and the mature neuronal marker MAP2 (red). In this example, neurons matured in BrainPhys™ Neuronal Medium show increased Synapsin 1 expression. Scale bar= 100 μm.
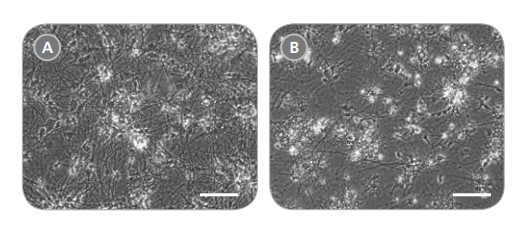
Figure 7. hPSC-Derived Neurons Generated in BrainPhys™ Neuronal Medium and NeuroCult™ SM1 and N2 Supplements are Healthy and Morphologically Normal
NPCs were generated from H9 cells using STEMdiff™ Neural Induction Medium in an embryoid body-based protocol. Next, NPCs were cultured for 44 DIV in (A) BrainPhys™ Neuronal Medium, supplemented with 2% NeuroCult™ SM1 Supplement, 1% N2 Supplement-A, 20 ng/mL GDNF, 20 ng/mL BDNF, 1 mM db-cAMP and 200 nM ascorbic acid to initiate neuronal differentiation, or (B) DMEM/F12 under the same supplementation conditions. Neuronal cultures differentiated from NPCs in BrainPhys™ Neuronal Medium display extensive neurite outgrowth and reduced cellular debris compared to cultures differentiated in DMEM/F12. Scale bar= 100 μm.
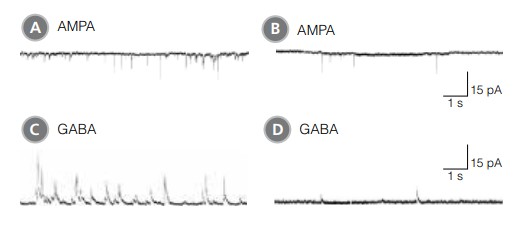
Figure 8. hPSC-Derived Neurons Matured in BrainPhys™ Neuronal Medium Show Improved Excitatory and Inhibitory Synaptic Activity
NPCs were generated from H9 cells using STEMdiff™ Neural Induction Medium in an embryoid body-based protocol. Next, NPCs were cultured for 44 DIV in (A,C) BrainPhys™ Neuronal Medium, supplemented with 2% NeuroCult™ SM1 Supplement, 1% N2 Supplement-A, 20 ng/mL GDNF, 20 ng/mL BDNF, 1 mM db-cAMP and 200 nM ascorbic acid to initiate neuronal differentiation, or (B,D) in DMEM/F12 under the same supplementation conditions. (A,C) Neurons matured in BrainPhys™ Neuronal Medium showed spontaneous excitatory (AMPA‑mediated; A) and inhibitory (GABA-mediated; C) synaptic events. The frequency and amplitude of spontaneous synaptic events is consistently greater in neuronal cultures matured in BrainPhys™ Neuronal Medium, compared to neurons plated and matured in DMEM/F12 (B,D). Traces are representative.
Product Information
Neuronal Media and Supplements for Culture and Maturation
| Product | Size | Catalog # |
|---|---|---|
| BrainPhys™ Neuronal Medium | 500 mL | 05790 |
| BrainPhys™ Neuronal Medium and SM 1 Kit * | 1 Kit | 05792 |
| BrainPhys™ Neuronal Medium N2-A/SM 1 Kit * | 1 Kit | 05793 |
| NeuroCult™ SM1 Neuornal Supplement (50X) | 10 mL | 05711 |
| N2 Supplement-A (100X) | 5 mL | 07152 |
| N2 Supplement-B (100X) | 5mL | 07156 |
*Kit includes basal medium plus supplements
References
1. Bardy C et al. (2015) Proc Natl Acad Sci 112 (20) E2725-E2734.
2. Brewer GJ et al. (1993) J Neurosci Res. 35(5):567-76.
3. Bottenstein JE (1985) Cell Culture in the Neurosciences: Current Topics in Neurobiology. New York: Plenum Press: 3-40.
Supplementary Reagents
Small Molecules for Neural Differentiation
| Molecule | Pathway/Target | Catalog # |
|---|---|---|
| Purmorphamine | Hedgehog pathway activator Activates smoothened | 72202 |
| All-Trans Retinoic Acid | Activates RAR | 72262 |
| Valproic Acid | Epigenetic modifier Inhibits histone deacetylase (HDAC1) | 72292 |
| CHIR99021 | WNT pathway activator Inhibits GSK3 | 72052 |
| DAPT | Notch pathway inhibitor Inhibits g-secretase | 72082 |
| SB431542 | TGF-b pathway inhibitor Inhibits ALK5, ALK4, ALK7 | 72232 |
| Forskolin | cAMP pathway activator Activates adenylyl cyclase | 72112 |
*selected products only, see www.stemcell.com/smallmolecules for more
Antibodies for Neural Differentiation
| Product | Host Species | Catalog # |
|---|---|---|
| Neuronal Class III beta-Tubulin | Mouse | 60052 |
| Anti-Human Nestin Antibody, Clone 10C2 | Mouse | 60091 |
| MAP2 Antibody, Clone AP20 | Mouse | 60049 |
| GFAP Antibody | Rabbit | 60128 |
*selected products only, see www.stemcell.com/antibodies for more
Cytokines for Neural Differentiation
| Product | Size | Catalog # |
|---|---|---|
| BDNF, Human, Recombinant | 5 μg | 02519 |
| bFGF, Human, Recombinant | 50 μg | 78003 |
| EGF, Human, Recombinant | 500 μg | 78006 |



