Comprehensive network activity made simple with Maestro MEA
Why measure neural activity?
In vitro neural models are a proven powerful strategy for the study of neural function and complex disorders of the human nervous system. The study of neurons has been historically complex, hindering advancements in human biology and treating disease. Maestro microelectrode array (MEA) technology makes it easy to measure electrical network behavior in live cells at high throughput, providing simple functional endpoints that capture biological complexity.
Neural network recordings
Axion BioSystems’ Maestro Pro and Maestro Edge provide a flexible, yet intuitive, assay of functional electrophysiology for neural applications. Axion’s MEA plates have a grid of tightly spaced electrodes embedded in the culture surface of each well. Neurons (orange) can be cultured over the electrodes (grey), and over time, as the cultures become established, neurons can form cohesive networks with an electrophysiological profile. The resulting electrical activity is captured from each electrode on a microsecond timescale providing both temporally and spatially precise data. The Maestro bioelectronic assay detects key parameters of neural network function, including activity, synchrony, and oscillation. All three endpoints are required for a complete understanding of functional neural networks.
THE MAESTRO ADVANTAGE
- It’s easy! Culture cells in each well, load the plate in the Maestro, and press record.
- Industry-leading electrode counts provide access to network-level information at scale.
- Multiwell MEA plates provide throughput flexibility to scale up functional assays.
- Noninvasive, label-free measurements monitor acute responses or long term development.
- Functional endpoints compliment existing assays used in drug screening, developmental biology, and disease modeling.
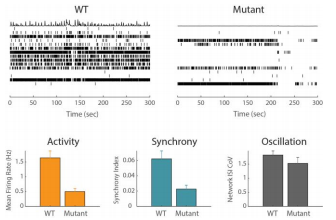
Example Application 1: iPSC disease modeling
Significant advancements in reprogramming basic human cells (induced pluripotent stem cells, iPSC) from both healthy and diseased donors into any cell type has laid the foundation for exploring human disease in vitro. In an iPSC-derived model of schizophrenia, the oscillation measure suggests there is little difference in neural networks in the mutant and wild type (WT) cells. However, the Maestro revealed a decrease in activity and synchrony in the mutant neurons relative to WT, an effect that would have been missed when looking at oscillation alone.
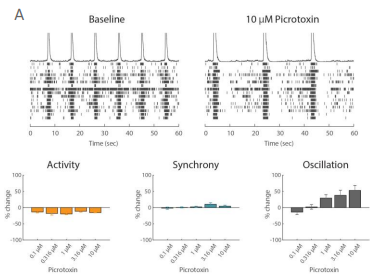
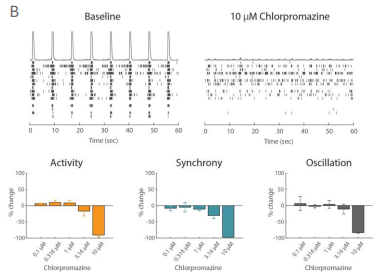
Example application 2: Neurotoxicology
Functional assessment is crucial to understanding potentially adverse effects on neuronal networks. The Maestro excels in preclinical studies to screen for unwanted seizurogenic activity. In iPSC-derived neurons, Picrotoxin, a neurotoxic, proconvulsant compound, caused an increase in oscillation in a dose-dependent manner, while having no effect on activity and synchrony. The antipsychotic medication, Chlorpromazine, had a very different effect, decreasing activity, synchrony, and oscillation at high concentrations. Biological complexity in neural networks is important, and all three of these measures are required to fully understand it. Maestro MEA makes it easy to capture and describe functional network behavior.