by Axion BioSystems
Data provided by Glycostem Therapeutics
Key Findings
>> Characterization of target antigen and CAR expression
>> Trastuzumab enhances potency of CD16+ NK cells
>> CAR NK-mediated cytotoxicity is specific to target antigen
>> Potency kinetics are ratio- and target cell-dependent
Abstract
Immunotherapies such as CAR T cells that have shown success in treating liquid tumors have had limited efficacy against solid tumors, including glioblastoma. Natural killer (NK) cells represent a promising approach for solid tumor treatment, and understanding mechanisms of NK-mediated cytotoxicity is important for cell therapy development. Here, we quantify kinetics of both antibody-dependent cellular cytotoxicity (ADCC) and CAR NK-mediated cytolysis against glioblastoma cells using the Maestro Z. Kinetics of these mechanisms are assessed across multiple target cell lines and effector:target ratios.
Introduction
Immunotherapy aims to harness and enhance the body’s immune system to fight cancer. Engineered autologous T cells expressing chimeric antigen receptors (CARs) have been remarkably successful in treating hematologic malignancies, including lymphoma, leukemia, and myeloma. CAR T cells have been less effective, however, for treating solid tumors.
Glioblastoma (GBM) is an aggressive astrocytoma with no effective treatments and a median survival rate of less than 15 months after diagnosis (Yu et al. 2021). Given the success of CAR T cells in treating liquid tumors, GBM has emerged as a new target for immunotherapy. Immunotherapies have been less successful against GBM, like other solid tumors, for several factors – primarily the immunosuppressive tumor microenvironment (TME) and difficulty identifying tumor-specific antigens for targeting (Yu et al. 2021). In addition to these challenges, GBM is highly heterogeneous, adding to difficulty surrounding target determination. Despite this, HER2 has emerged as a promising target in preclinical studies and early phase clinical trials (Yu et al. 2021).
As an alternative to CAR T cells, natural killer (NK) cells represent a newer approach to cancer immunotherapy. NK cells have shown efficacy in preclinical studies for GBM and other tumors. Moreover, NK cells do not have the same HLA compatibility concerns as T cells, providing increased potential for an allogeneic “off-the-shelf” therapeutic (Zhang et al. 2016). Several mechanisms of NK cell immunotherapy are being explored, including antibody-dependent cellular cytotoxicity (ADCC) and CAR NK cells. ADCC is a physiological mechanism mediated by NK cells to induce cancer cell death. Both enhancing ADCC via antibody treatment and CAR NK cell therapy represent promising strategies for NK cell-mediated immunotherapy.
In this study, we demonstrate a quantitative in vitro assay to evaluate NK-mediated cytotoxicity of glioma cells. The Maestro Z was used to assess both ADCC and CAR NK-mediated cytotoxicity of two glioblastoma cell lines expressing HER2. Trastuzumab, a HER2-binding antibody, and HER2-targeted CAR NK cells were evaluated to quantify the kinetics of these mechanisms.
Results
Characterization of target antigen and CAR expression
For CAR NK-mediated killing and efficacy of ADCC, the target cells must express sufficient amounts of target antigen. In these experiments, both CAR NK cells and trastuzumab, the antibody used to elicit ADCC, were used to target HER2 on two glioblastoma (GBM) target cell lines – A172 and LN229. Flow cytometry was used to assess HER2 expression on both target cell lines (Fig. 1), with A172 cells moderately expressing HER2 and LN229 cells highly expressing HER2.
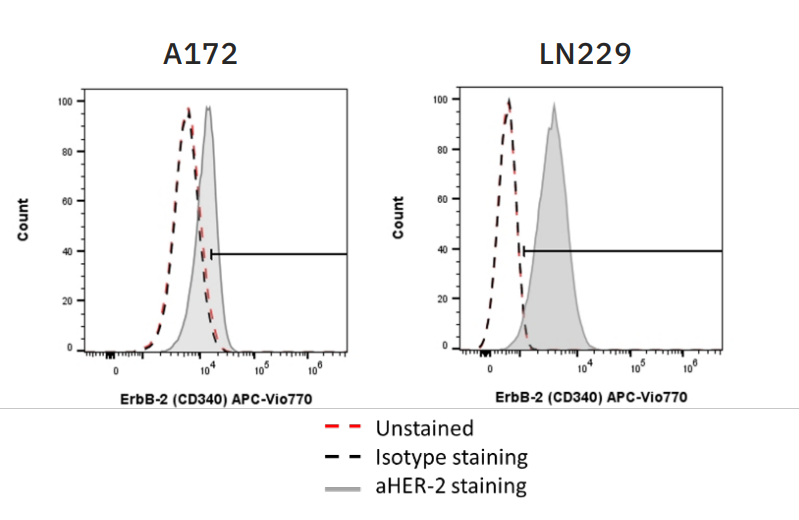
Flow cytometry was also used to confirm CAR expression on CAR NK cells and CD16 expression on CD16+ NK-92 cells (data not shown). CAR expression was 74.2% on HER2 CAR-transduced NK-92 cells and 86.69% on CD19 CAR-transduced NK-92 cells. CD16 expression was 90% on CD16+ NK-92 cells.
To equilibrate CD16 expression with CAR expression, CD16+ NK-92 cells were diluted with NTD NK-92 cells to match CAR expression levels prior to cytotoxicity assays.
Trastuzumab enhances potency of CD16+ NK cells
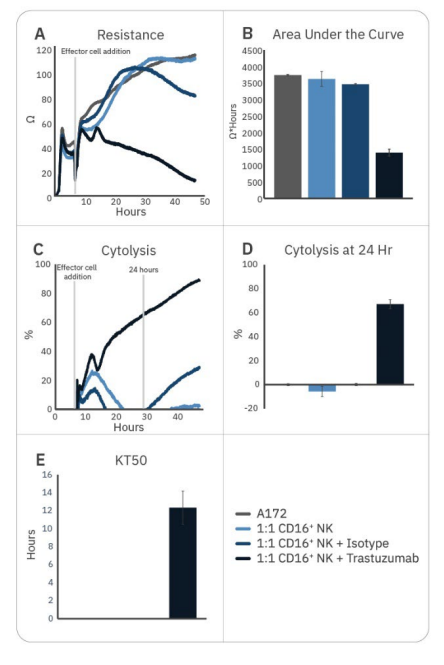
Natural killer cell potency can be enhanced by various strategies, including antibody treatment to induce ADCC. While CD16+ NK-92 cells alone were not effective against A172 cells, the addition of trastuzumab – which binds to HER2 expressed on the A172 cells – significantly enhanced CD16+ NK-92 cell potency, as measured by decreased resistance, increased cytolysis, and faster KT50 (Fig. 2).
Trastuzumab addition resulted in 50% cancer cell death by 12 hours and approximately 70% by 24 hours. By contrast, adding isotype IgG had little effect on CD16+ NK92 cell-mediated cytotoxicity, providing evidence that the difference seen with trastuzumab addition is due to ADCC.
CAR NK-mediated cytotoxicity is specific to target antigen
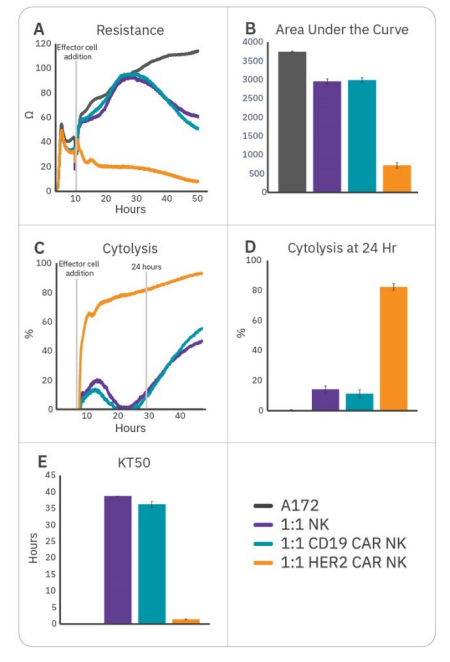
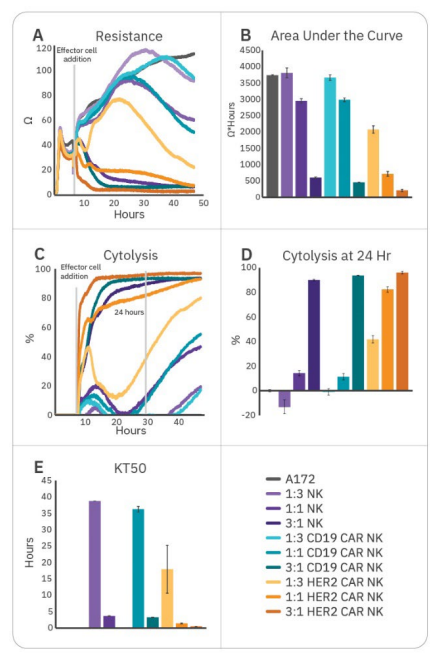
To further enhance cell potency, natural killer cells may be transduced with CAR constructs. HER2-targeted and CD19-targeted CAR NK cells were compared to non-transduced NK cells to demonstrate antigen selection. After 6 hours of tumor cell growth, NK cells were added and immune cell-mediated killing was observed, as indicated by a decrease in resistance (Fig. 3A). Percent cytolysis (Fig. 3B) was alculated as described in the Methods using non-treated A172 cells as a control. Overall tumor growth was quantified by area under the curve (Fig. 3C), and NK cell potency was measured both by % cytolysis 24 hours post-effector cell addition (Fig. 3D) and kill time 50 (Fig. 3E).
CAR NK cells demonstrated varying levels of cytotoxicity depending on the CAR construct and target antigen. Non-transduced NK-92 (Fig. 3, purple) and CD19 CAR NK cells (Fig. 3, teal) demonstrated mild efficacy, inducing 10-15% cytolysis at 24 hours and taking 35-40 hours to induce 50% cytolysis. As A172 cells do not express CD19, these groups reflect non-specific, baseline NK-92 potency. HER2 CAR NK cells (Fig. 3, orange) were significantly more cytototoxic, killing 50% of the target cells within 1.5 hours and 80% by 24 hours. This reflects higher potency for the HER2-targeted CAR NK cells, suggesting antigen recognition and specificity is an important determinant for CAR NK cell potency.
Potency kinetics are ratio- and target cell-dependent
An ideal in vitro assay can help detect differences between therapeutic strategies and evaluate critical steps in development. Testing a range of effector:target ratios ensures an appropriate dynamic range for making meaningful comparisons. To demonstrate the ability of the assay to distinguish between E:T ratios, effectors were added at three different E:T ratios (1:3, 1:1, and 3:1). While all groups effectively killed A172 cells at 3:1, only HER2-targeted CAR NK cells demonstrated substantial killing at all three ratios (Fig. 4). Little (<20%) or no cytotoxicity was observed at 1:3 and 1:1 ratios for CD16+ NK-92, NTD, and CD19 CAR NK groups at 24 hours. This both suggests higher potency for HER2-targeted CAR NK cells, as well as demonstrates the importance of E:T ratio selection when assessing potency and kinetics of cytotoxicity. At particularly high or low E:T ratios, dynamic range to detect differences in potency may be limited and effects may be missed.
Target cell line is also an important consideration for potency assays, as various target lines will have varying antigen expression levels and susceptibility. To demonstrate this, the ADCC and CAR NK-mediated killing assays described above were repeated on LN229 cells, an alternative HER2-expressing glioblastoma cell line (Fig. 5). While enhanced potency was also observed via trastuzumab or HER2 CAR NK addition compared to relevant controls, there were some differences.
Notably, the LN229 cells were susceptible to all groups, including the CD16+ NK-92 cells (Fig. 5, navy) which were not effective against the A172 cells (Fig. 2-4). Additionally, killing was significantly faster, with most killing occurring in <6 hours (Fig. 5B, D) and KT50 values ranging from 1 hour (HER2 CAR NK; Fig. 5E, orange) to approximately 5 hours (CD16+ NK-92 with or without isotype IgG; Fig. 5E, light navy and navy, respectively). Finally, both NTD and CD19 CAR NK cells effectively killed the LN229 cells, reflecting inherent susceptibility of the LN229 cells compared to the A172 cells (Fig. 5, purple and teal, respectively). These results highlight the importance of selecting an appropriate target line for potency assays.
Conclusion
The Maestro Z allows for label-free, real-time monitoring of immune cell-mediated cytotoxicity of cancer cells, allowing for quantitative immune cell potency assessment in vitro. As NK cells have emerged as a promising cell therapy, methods to evaluate potency across various mechanisms of cell killing, such as ADCC and CAR NK-mediated cytotoxicity, are vital for immunotherapy development and manufacturing. Multiple approaches are needed to develop effective therapeutics for glioblastoma and other solid tumors. Here, we demonstrate the ability to distinguish kinetics among ADCC and CAR NK-mediated killing, different target cell lines, and varying effector:target ratios. By assessing and accounting for these factors, we highlight the utility of the assay for the development and testing of potential NK cell immunotherapies to treat solid tumors
Materials and Methods
Cells and reagents
A172 (Cat. CRL-1620), LN229 (Cat. CRL-2611), and NK-92 (Cat CRL -2407) cells were obtained from ATCC (Manassas, VA). NK-92 variants expressing CD16 or CAR transgenes were generated by lentiviral transduction. A172 and LN229 were cultured in Dulbecco's Modified Eagle's Medium (ATCC, Cat. 30-2002) with 10% FBS (Gibco, Cat. 16000044). Non-transduced NK-92 cells and CD16- or CAR-expressing NK-92 cells were cultured in Glycostem basal growth media (GBGM, Glycostem, Kloosterstraat, The Netherlands), 10% FBS and 1000 U/mL IL-2 (Proleukin, VU Medisch Centrum). CD16 and CAR expression were measured on NK cells by flow cytometry before starting the assay.
Antibodies used: anti-HER2 (anti-ErbB-2 (CD340)-APC-Vio770, clone 4D2, Miltenyi, Cat. 130-106-756), mouse IgG1-APC-Vio 770 (isotype control, clone IS5-21F5, Miltenyi, Cat. 130-113-759), Trastuzumab (Bioconnect, Begonialaan, The Netherlands, Cat. HY-P9907), human IgG isotype control (Thermo Fisher, Waltham, MA, Cat. 02-7102), mouse anti-human IgG (H+L) AF488 (Thermo Fisher, Cat. A11013), anti-human CD16 (BioLegend, San Diego, CA, Cat. 302050).
Flow cytometry
To determine HER2 expression on A172 and LN229 cells, 100,000 cells/well were incubated with anti-HER2 antibody (Miltenyi Biotec, 130-106-756) (1:50) or isotype control (Miltenyi Biotec, 130-104-618) (1:50) and 7-AAD (Sigma, A9400) to mark dead cells. Cells were then resuspended in 200 µL 7-AAD containing FACS buffer (196 µL buffer + 4 µL from 0.1mg/ml 7-AAD stock) and flow cytometry was run. Flow cytometry was performed to determine HER2 expression, and gates were drawn to exclude doublets and dead cells.
Maestro Z assay platform
The Maestro Z platform (Axion BioSystems) uses impedance measurements (ohms, Ω) to quantify the presence of cells on electrodes embedded in the bottom of the wells of CytoView-Z plates (Axion BioSystems). Cellular impedance is a well-established technique for measuring cell attachment, spreading, proliferation, coupling, membrane integrity (cell death), and subtle changes in cell conformation. Detection is noninvasive and label-free, so it can quantify dynamic cellular responses over minutes, hours, and days. The Maestro Z's built-in environmental chamber finely controls temperature and CO2, ensuring a consistent, optimal experimental environment.
NK-92-mediated ADCC assay
To measure NK-92 cell-mediated killing, A172 or LN229 cells were cultured as described above. After taking a media reference measurement (50 µL media/well), 30,000 A172 or 40,000 LN229 target cells were seeded in 40 µL/well and incubated in the Maestro Z for 5 hours at 37°C and 5% CO2.
Trastuzumab (Bioconnect, HY-P9907) or isotype control (Thermo Fisher Scientific, 02-7102) were added to the target cells at a final concentration of 10 µg/mL in 10 µL/well and incubated for 15 minutes. NK-92 effector cells were added to the target cells at E:T ratios of 1:1 and 1:3 in 100 µL/well, for a total well volume of 200 µL. E:T ratios were based on CD16+ effectors.
The plate was then returned to the Maestro Z and impedance was measured for 40-100 hours.
CAR NK-mediated cytotoxicity assay
To measure CAR NK cell-mediated killing, A172 or LN229 cells were cultured as described above. After taking a media reference measurement, target cells were seeded in 40 µL/well and docked on the Maestro Z for 6 hours at 37°C and 5% CO2.
HER2 CAR NK, CD19 CAR NK, or non-transduced (NTD) NK effector cells were added to the target cells at E:T ratios of 1:3, 1:1 and 3:1 in 100 µL/well, for a total well volume of 200 µL. E:T ratios were based on CAR+ effectors.
The plate was then returned to the Maestro Z and impedance was measured for 40-100 hours.
Calculation of cytolysis
%Cytolysis was used to quantify cell death and was calculated using the following equation:
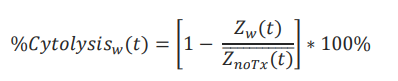
where 𝑍noTx(𝑡) is a time series and the mean of the no treatment control wells.
%Cytolysis, kill time 50 (KT50), and area under the curve (AUC) of resistance or cytolysis were calculated and exported using AxIS Z software.
About Glycostem Therapeutics
Glycostem Therapeutics is focused on the development of stem cell-derived Natural Killer cells (NK cells) as a medicinal asset in the fight against cancer. NK cells are the new star in the domain of cellular immunotherapy; due to their tightly regulated “natural killing” of cancer cells, they play an important role in control and even cure of both solid and hematological malignancies, like Acute Myeloid Leukemia (AML) and Multiple Myeloma (MM).
References
- Yu MW and Quail DF (2021) Immunotherapy for Glioblastoma: Current Progress and Challenges. Front. Immunol. 12:676301. doi: 10.3389/fimmu.2021.676301
- Zhang C, Burger MC, Jennewein L, Genßler S, Schönfeld K, Zeiner P, Hattingen E, Harter PN, Mittelbronn M, Tonn T, Steinbach
- JP, Wels WS (2016). ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. J. Natl. Cancer Inst., Volume 108, Issue 5, May 2016, djv375, https://doi.org/10.1093/jnci/djv375
Authors
Anna-Maria Georgoudaki, Adil D. Duru, Didem Ozkazanc, Jan Spanholtz Glycostem Therapeutics Research Department, Oss, Netherlands
Austin Passaro, Stacie Chvatal, Axion BioSystems, Atlanta, GA