Key Findings
>> Docetaxel inhibits threatening cancer cell characteristics in Calu3 cells.
>> The Omni Pro 12 improves assay efficiency.
>> Multi-assay integration in the Omni Pro 12 can enhance drug profiling.
Abstract
This study investigates the toxicity profile of docetaxel, a chemotherapeutic agent, on lung cancer cells, specifically assessing its effects on cell viability, migration, and colony-forming ability. Using the Omni Pro 12 high-throughput live-cell imaging system, multiple 2D in vitro assays were conducted simultaneously, enabling a comprehensive evaluation of docetaxel’s impact on cancer cell behavior. The results demonstrated that docetaxel decreases the viability, proliferation, and migration of Calu3 cells in a dose-dependent manner, though its effectiveness varied across different assays.
Introduction
The field of oncology focuses on studying the development and progression of cancer and its potential treatments. Oncology researchers aim to achieve a deeper understanding of the underlying causes of cancer, develop new diagnostic tools, and investigate more effective therapeutic drugs. To assess drug sensitivity and resistance, various in vitro assays are utilized, including cell cytotoxicity, migration, and clonogenic assays1,2. Docetaxel, a chemotherapeutic agent that inhibits microtubule depolymerization, is one of the drugs extensively studied for its toxicity profile3.
The toxicity profiles of drugs are highly dependent on the type of assay performed due to differences in methods, sensitivity, and readout, as well as whether a drug affects cell death, proliferation, or migration. Employing a combination of assays can yield a more comprehensive and accurate drug toxicity profile, which is crucial for reducing false outcomes in clinical stages.
Utilizing the Omni Pro 12, a high-throughput live-cell imaging system, significantly accelerates the process of drug toxicity profiling. This system allows for multiple assays to be run simultaneously, thus streamlining experimental workflows in an automated fashion. Furthermore, this approach minimizes variability in results by maintaining a consistent experimental environment across all assays, thereby improving the reliability and reproducibility of the data.
In this study, we conducted a comprehensive assessment of the toxicity profile of docetaxel by performing a cytotoxicity, cell migration, and colony formation assay. By analyzing data from these assays, we aimed to gain a thorough understanding of the impact of docetaxel on key aspects of cancer progression, namely cell viability, migration, and colony-forming potential4. This provides valuable insights into its potential therapeutic efficacy and the importance of integrating multiple assays to better predict drug responses.
Materials and Methods
Cells and reagents
Calu3 (Colorectal Adenocarcinoma; ATCC, Cat. HTB-55) cells were maintained in DMEM (Gibco, Cat. 41965-039) supplemented with 10% fetal bovine serum (FBS; Gibco, Cat. 16000044) and 1% Penicillin/Streptomycin (P/S, Gibco, Cat. 15140122) referred to as complete DMEM. Cells were passaged twice per week using a 1:4 ratio.
Omni imaging platform
The Omni Pro 12 system allows users to perform up to 12 single experiments with custom experimental settings (time interval and software analysis) simultaneously. Figure 1 shows an overview of all assays performed with the Omni Pro 12 in this study to investigate the effect of docetaxel on Calu3 cells.
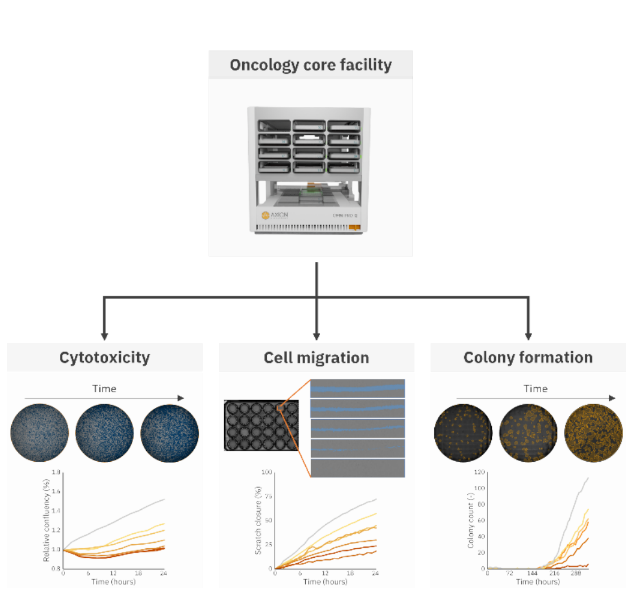
Cytotoxicity assay
The effect of docetaxel on the proliferation and viability of Calu3 cells was investigated through a cytotoxicity assay. Calu3 cells were seeded at 50,000 cells/cm2 density in a well plate and placed in the Omni Pro 12 to monitor the confluency over time. Calu3 cells were grown until approximately 50% confluence. Upon reaching 50% confluency, the Omni Pro 12 software automatically sent a notification email after which docetaxel was added. Docetaxel was added at concentrations of 50 nM, 100 nM, 250 nM, 500 nM, 750 nM, and 1000 nM, except in the control group (N=4 per group). Subsequently, the impact of docetaxel on the Calu3 cell culture was monitored over 24 hours, with images taken at 2- hour intervals, using the Omni Pro 12. The confluency levels during the experiment were assessed using the Confluency Module for quantitative data analysis.
Scratch assay
The effect of docetaxel on the migration of Calu3 cells was investigated through a cytotoxicity assay. Calu3 cells were seeded onto a 24-well plate at a density of 100,000 cells/cm2 and allowed to reach 80-90% confluence as determined by the Confluency Module. A scratch was created across the cell monolayer using a 200 μL pipette tip. Following scratch induction, the cells were gently washed with phosphate-buffered saline (PBS), and fresh medium containing varying concentrations of docetaxel (0 nM, 50 nM, 100 nM, 250 nM, 500 nM, 750 nM, and 1000 nM), was added (N=4 per group). Subsequently, the cells were imaged over 24 hours with a time interval of 1 hour using the Omni Pro 12. Automated analysis of the scratch area was performed utilizing the Scratch Assay Module to quantify the extent of wound closure over time.
Clonogenic assay
The effect of docetaxel on the clonogenicity of Calu3 cells was investigated through a clonogenic assay. Calu3 cells were seeded in a 6- well plate at a density of 50,000 cells per well in complete DMEM medium. Hourly scans were conducted using the Omni Pro 12 imaging system. Upon reaching 70% confluency, the cells were washed twice with phosphate-buffered saline (PBS) and treated with varying concentrations of docetaxel (10 nM, 25 nM, 50 nM, 100 nM, and 250 nM), except for the control group (N=3 per group). Following a 1-hour incubation period, the cells were rinsed twice with PBS, trypsinized, and counted using the Exact FL cell counter (Axion Biosystems). The treated cells were subsequently seeded into individual wells of a 6-well plate at a density of 500 cells per well and imaged for 324 hours using the Omni Pro 12 with a time interval of 6 hours.
The number of colonies was determined using the Clonogenic Assay Module and the survival fraction was determined following procedures like those outlined in our previous application note 'Quantifying chemotoxicity on cancer cell colony formation using live-cell imaging'.
Results
Docetaxel inhibits cell proliferation of Calu3 cells
The lung cancer cell line, Calu3, typically grows in dense clusters and docetaxel was shown to exhibit a cytotoxic effect on cells mainly at the periphery, also on top, of these clusters, while cells in the center appeared to be protected by the peripheral cells (see arrows in Figure 2A).
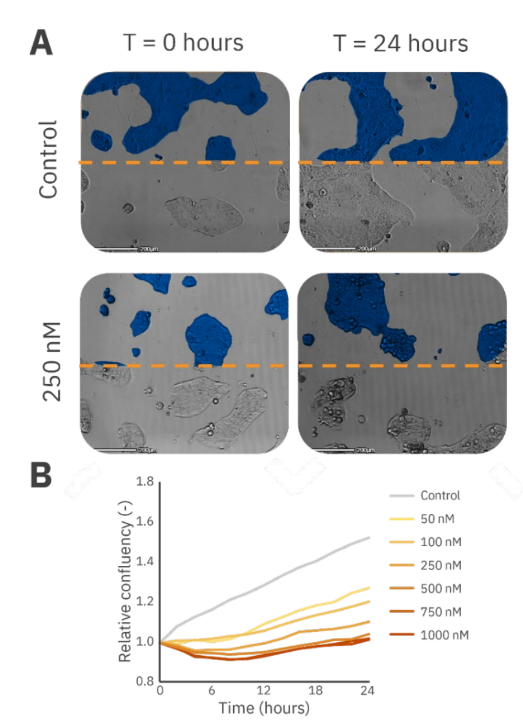
Docetaxel had an immediate toxic effect on Calu3 cells, and after 10 hours the dose-dependent response of Calu3 cell proliferation to different concentrations of docetaxel was observed. This dose-dependency became more evident when examining the cells’ recovery capacity over 24 hours. Cells treated with higher concentrations of docetaxel were unable to recover as effectively as those treated with lower concentrations (Figure 2B).
The migration speed of Calu3 cells decreased upon higher concentrations of docetaxel
Although the well-known scratch assay requires some optimization for Calu3 cells, as they grow in tight clusters which decreases consistency in the scratch, it is often used to analyze the effect of chemotactic drugs on cell migration in cancer-affected epithelial tissue. Using Omni Pro 12 with the Scratch Assay Module, these inconsistencies did not affect the outcome since the entire scratch was detected (Figure 3A).
Using this setup, a dose-dependent effect of docetaxel on the migration of Calu3 cells was observed (Figure 3B). The percentage of scratch closure after 24 hours decreased with an increase in the concentration of docetaxel.
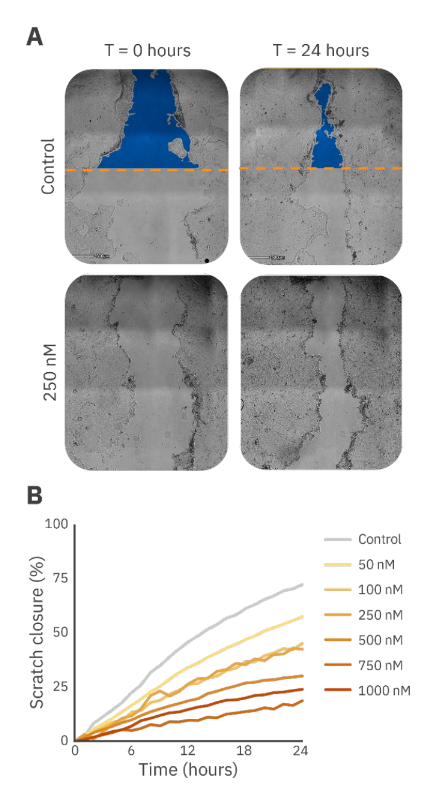
Comparable to the results of the cytotoxicity assay, Calu3 cell migration showed to have a dose-dependent response to docetaxel. However, the lower concentrations of docetaxel seemed to have a higher impact on the proliferation compared to the migration of Calu3 cells shown by a larger impact of 50 nM docetaxel. The results of the scratch assay showed a more gradual effect compared to the cytotoxicity assay results (Figures 2B and 3B).
The colony-forming capacity of Calu3 cells decreased upon higher concentrations of docetaxel
The effect of docetaxel on the proliferation capacity of single Calu3 cells was determined by counting the number of colonies formed over time. After approximately 2 weeks, the total number of colonies was used to measure the survival fraction.
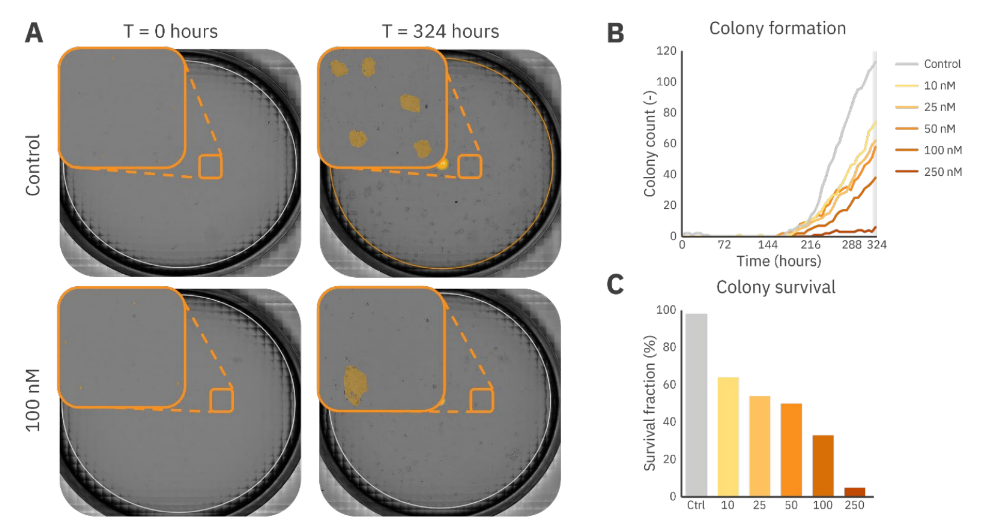
As shown in Figure 4A, docetaxel impacted the single-cell proliferation capacity which is shown by a decreased number of cells that multiplied and formed colonies after 324 hours when adding 100 nM docetaxel (Figure 4A). Figures 4B and 4C show the dose-dependent behavior of the colony-forming capabilities of Calu3 cells, where the highest concentration resulted in minimal colony formation in 2 weeks of growth. Additionally, Figure 4B shows the importance of the long-term assay as these cancer cells required 180 hours at least to proliferate enough to form colonies (minimal size: 1.0 x 105 µm2).
Conclusion
This study focused on the effect of docetaxel on various aspects of lung cancer cells. The results, both images and quantitative data, demonstrate that docetaxel effectively inhibits Calu3 cancer cell viability, proliferation, and migration in a dose-dependent manner, however, the effectiveness of docetaxel varied by concentration across different assays. The use of multiple in vitro assays all performed simultaneously using the Omni Pro 12 provided a comprehensive toxicity profile, which offers valuable insights into the potential therapeutic efficacy of docetaxel in lung cancer treatment. This study underscores the importance of integrating multiple assays to better predict drug responses and as shown by the use of the Omni Pro 12, to ensure similar experimental conditions during all assays.
References
1. Hoffman, R. M. (1991). In vitro sensitivity assays in cancer: a review, analysis, and prognosis. Journal of clinical laboratory analysis, 5(2), 133-143.
2. Varankar, S. S., & Bapat, S. A. (2018). Migratory metrics of wound healing: a quantification approach for in vitro scratch assays. Frontiers in Oncology, 8, 633.
3. Pienta, K. J. (2001, August). Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer. In Seminars in oncology (Vol. 28, pp. 3-7). WB Saunders.
4. Hou, Y., Zhao, C., Xu, B., Huang, Y., & Liu, C. (2021). Effect of docetaxel on mechanical properties of ovarian cancer cells. Experimental Cell Research, 408(1), 112853.
Authors
Linda Boekestijn, Application Scientist II
Lieke Stemkens, Senior Application Scientist
Axion BioSystems, Eindhoven, The Netherlands