Culturing Spheroids
-
Thaw and culture cancer cells in accordance with supplier recommendations and passage as needed.
-
Remove flask(s) of cells from the incubator, aspirate the media, and rinse with PBS. Using trypsin, or another cell dissociating agent, detach and collect the cells from the flasks per reagent recommendations.
-
Remove a sample of the cell suspension and count the cells using a hemocytometer to determine the total number of viable cells.
-
Transfer the cell suspension to a 15 mL conical tube and centrifuge to a pellet. Aspirate the supernatant, being careful not to disturb the cell pellet.
-
Dilute the cell suspension in complete medium to a working concentration of cells per 200 µL.
-
Transfer 200 µL of the cell suspension to each well of a ultra-low attachment (ULA) U-bottom plate to achieve the desired number of cells per spheroid.
Tip: For non-ULA plates:- Add 200 µL of STEMCell Technologies™ Anti-Adherence Rinsing Solution to each well.
- Centrifuge at 200 x g for 5 minutes.
- Aspirate and wash with PBS.
- Add 200 µL of PBS to each well.
- Aspirate the PBS when the cell suspension is ready to transfer.
-
Centrifuge the plate at 200 x g for 5 minutes then place in the incubator at 37°C and 5% CO2.
Tip: use “mid-low” speed for centrifuge deceleration to avoid cell spreading against the walls of the wells. -
Allow the spheroids to grow for 4 days.
Preparation of CytoView-Z Plate and baseline recording
-
After 4 days of spheroid culture, add 100 µL of extracellular matrix (ECM) to the CytoView-Z 96 plate.
Tip: Reconstitute ECM as recommended by supplier. Store as single use aliquots for more consistent results. -
Incubate the plate at 37°C and 5% CO2 for at least 1 hour.
-
Aspirate the excess ECM from the plate.
-
Add 100 µl of complete medium to the wells of the plate and 8 mL of sterile water to the on-plate reservoirs to increase humidity.
-
Dock the plate in a Maestro platform to measure the media-only baseline. Transfer the plate to a biosafety cabinet when the baseline is complete.
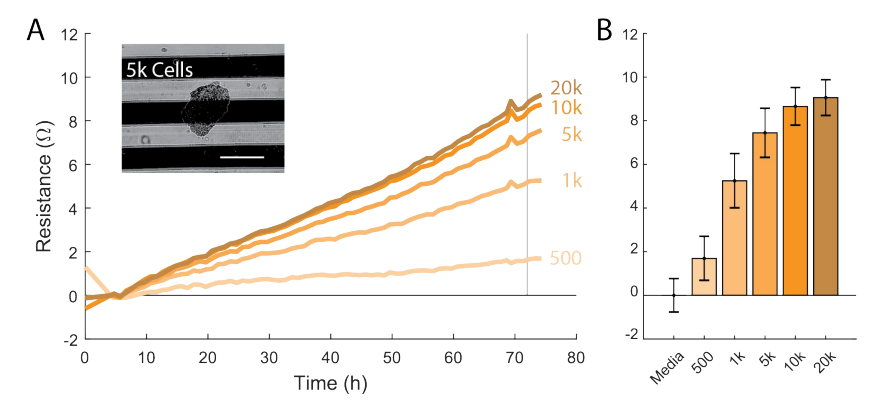
Transfer spheroids to CytoView-Z Plate
-
Check the spheroids under a microscope for stability and cell-to-cell adhesion. The spheroids should have a defined shape (e.g., no single cell scatter).
-
Insert a P-1000 micropipette with wide bore tips into the U-bottom well and make sure the tip is touching the bottom of the well. The tip should surround the spheroid.
-
Pipette up and down a few times to loosen the spheroid from the U-bottom well.
-
With the tip still touching the bottom, pipette up 100 µL from the U-bottom well and transfer the media with the spheroid into the well of the Cytoview-Z plate.
Note: For multiple spheroids per well, combine spheroids into a single U-bottom well. Wait for each spheroid to settle to the bottom. Then, pipette all the spheroids in 100 µL and transfer to the Cytoview-Z plate.Tip: Periodically check if the transfer was successful. If there is no spheroid in the Cytoview-Z well:
- Check the coordinating U-bottom well for the spheroid(s).
- Repeat steps 15-17.
- Re-check the U-bottom and Cytoview-Z wells under a microscope.
-
Dock the plate into the Maestro platform.
Tip: Cytoview-Z wells include a viewing window. If spheroid(s) land in the area without electrodes (dark bands), it is advised to disable that well in Axis Z.
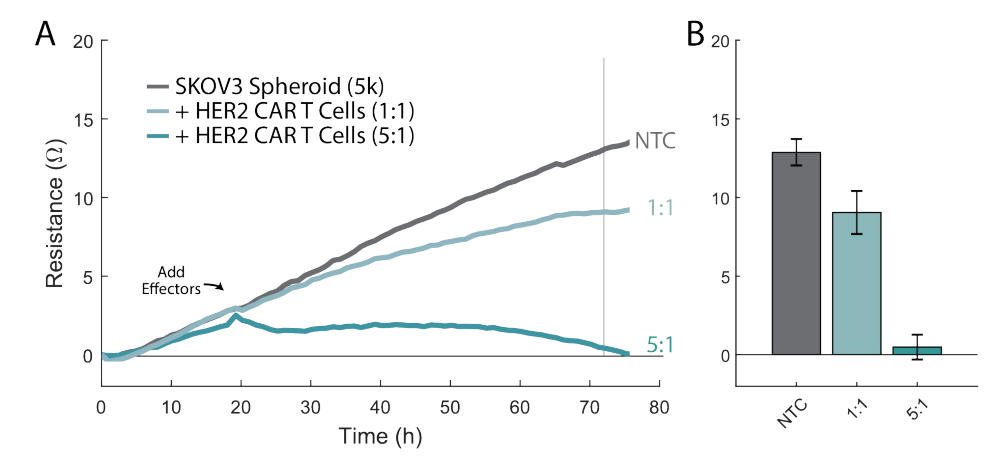
Required materials
Consumables
| Item | Vendor | Catalog # |
|---|---|---|
| CytoView-Z 96 Plate | Axion BioSystems | Z96-IMP-96B |
| Extracellular matrix | Various | |
| Gibco™ Trypsin-EDTA (0.5%) | Fisher Scientific | 15-400-054 |
| Gibco™ PBS, pH 7.4 | Fisher Scientific | 10-010-031 |
| 1000 µl Wide Bore Pipette Tips | Various | |
| ULA U-Bottom Plates | Corning | 4515 |
| Cell Culture Media | Various | |
| 15 mL Centrifuge Tubes | Various |
Equipment
| Item | Vendor |
|---|---|
| Maestro Edge, Pro, Z, or ZHT | Axion BioSystems |
| AxIS Z | Axion BioSystems |
| Biological Safety Cabinet | Various |
| Cell Culture Incubator | Various |
| Hemocytometer or Cell Counter | Various |
| Phase Contrast Microscope | Various |
| Tabletop Centrifuge | Various |
| 37°C Water Bath | Various |