Poster initially presented at SPS 2021
MEA Assay with Cardiomyocytes
Structure and Function in One Assay
The Maestro MEA platform enables assessment of in vitro cardiomyocyte function and structural integrity with an easy-to-use benchtop system. The Maestro detects electrical signals from cells cultured onto an array of planar electrodes in each well of the MEA plate, with five modes providing critical safety information:
Field Potential Provides Sensitive, Label-Free Measurements of Acute and Chronic Function
- Field Potential – “gold standard” measurement for multiwell cardiac electrophysiology.
- LEAP – first truly scalable technique for acquiring action potential signals from intact cardiac monolayers.
- Conduction – detect speed and direction of action potential propagation.
- Contractility – assess the contractility of cardiac monolayers adhered to the 2D array.
- MEA Viability – track cell coverage and viability using impedance technology.
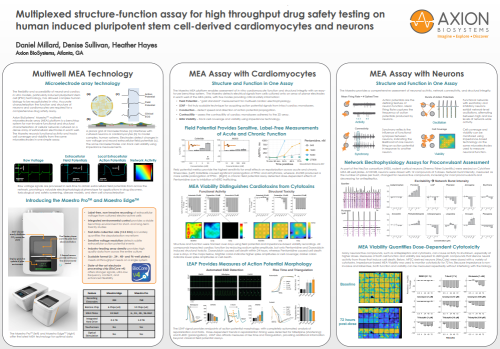
Field potential metrics provide the highest sensitivity for small effects on repolarization across acute and chronic timescales. (Left) Dofetilide caused significant prolongation of FPDc and arrhythmias, whereas JNJ303 produced a more subtle prolongation of FPDc. (Right) A chronic field potential assay detected dose-dependent effects of Pentamidine due to inhibition of hERG trafficking.
MEA Viability Distinguishes Cardiotoxins from Cytotoxins
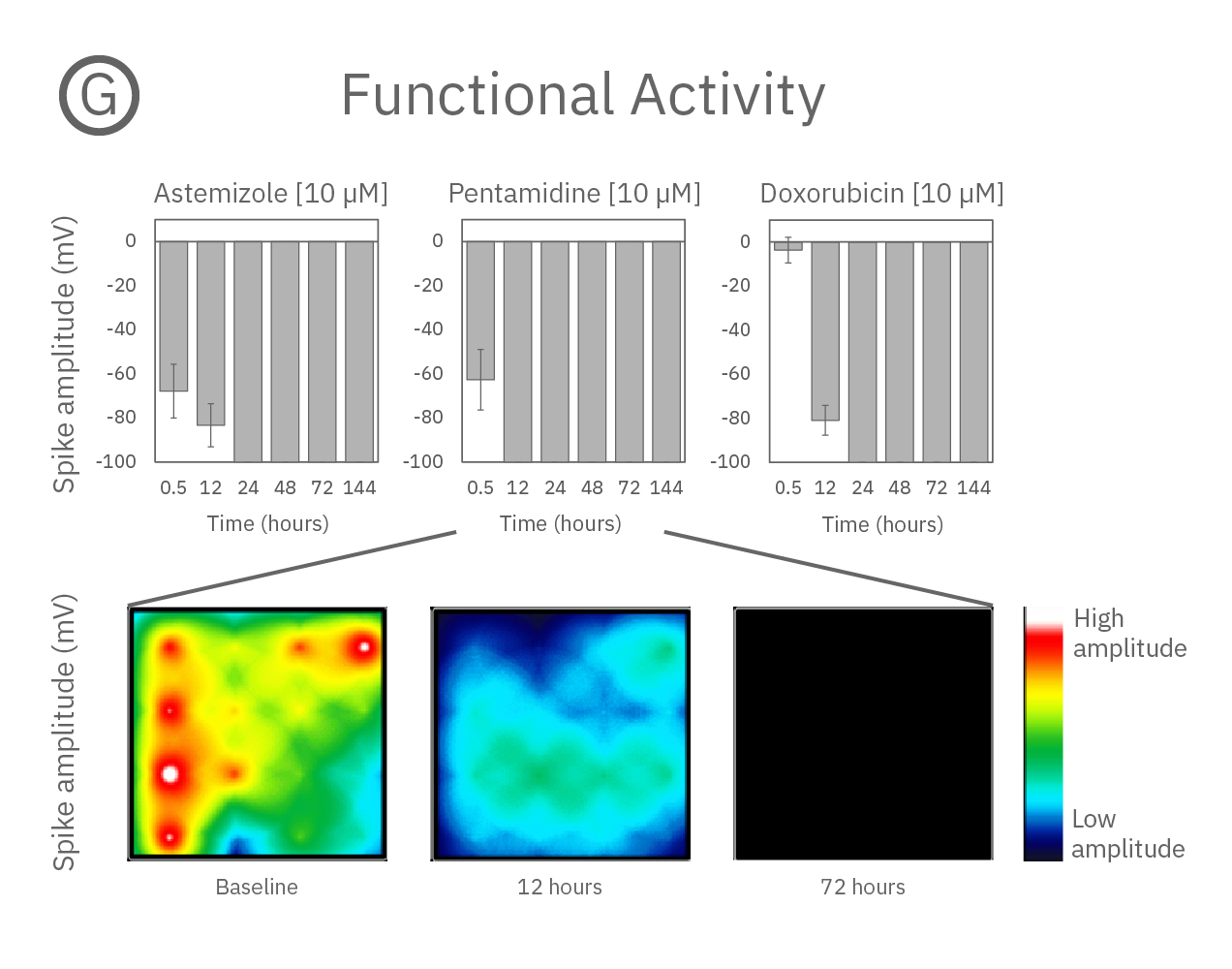
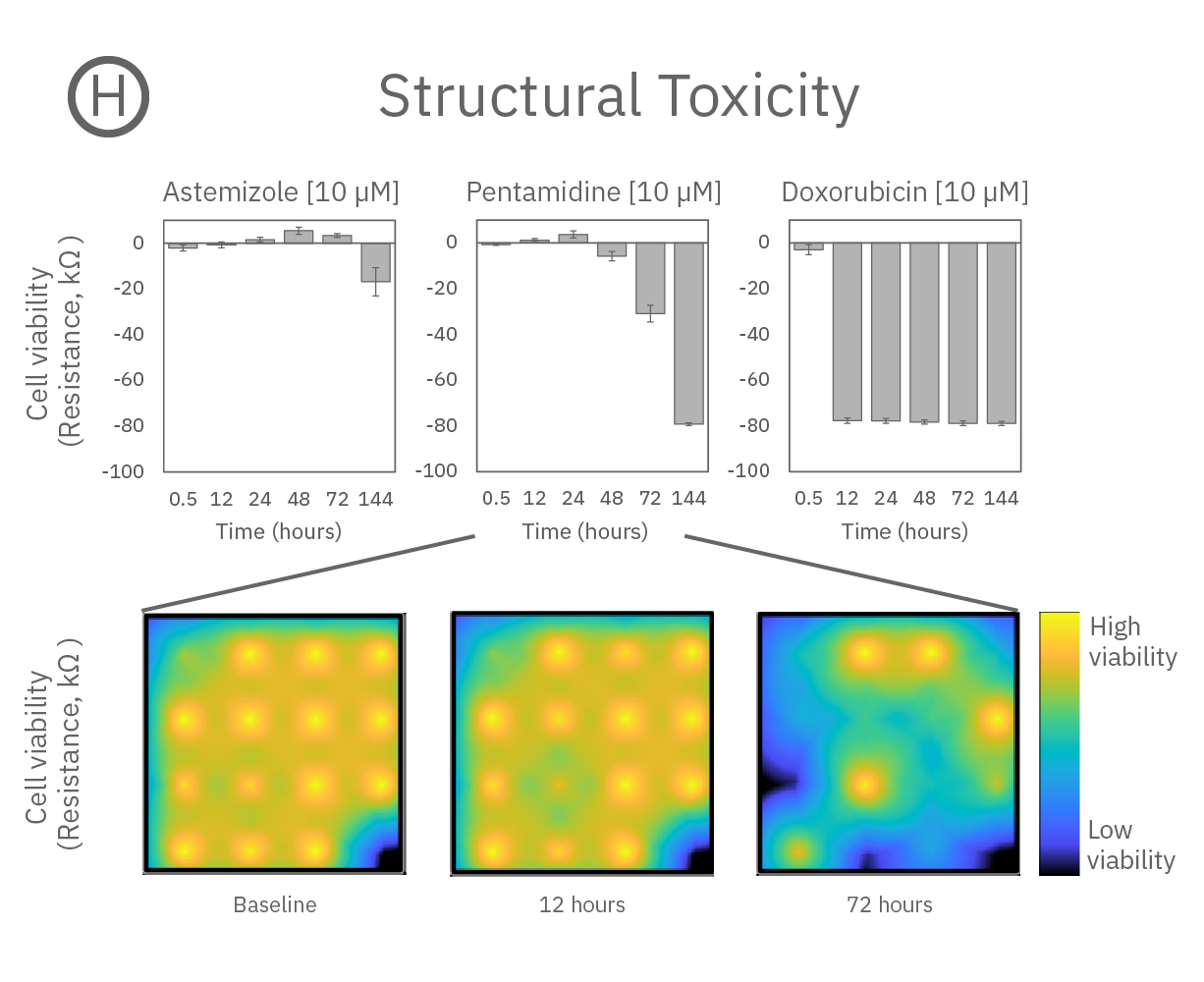
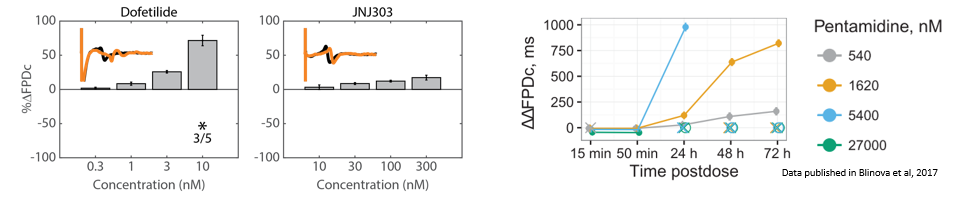
Structure and function were tracked over days using field potential and impedance-based viability recordings. All compounds impacted cardiac function by reducing sodium spike amplitude, but only Pentamidine and Doxorubicin induced structural toxicity. Doxorubicin caused cell death within 24 hours, whereas Pentamidine caused cell death over 6 days. In the maps below, brighter colors indicate higher spike amplitudes or cell coverage; darker colors indicate lower spike amplitudes or cell death.
LEAP Provides Measures of Action Potential Morphology
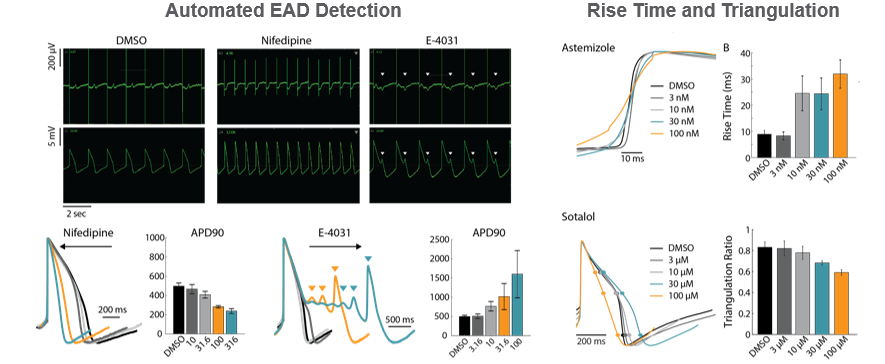
The LEAP (local extracelluar action potential) signal provides endpoints of action potential morphology, with completely automated analysis of repolarization and EADs. Dose-dependent trends in repolarization timing were detected for Nifedipine (shortening) and E-4031 (prolongation). LEAP also affords measures of rise time and triangulation, providing additional information beyond classical field potential assays.
MEA Assay with Neurons
Struction and Function in One Assay
The Maestro provides a comprehensive assessment of neuronal activity, network connectivity, and structural integrity.
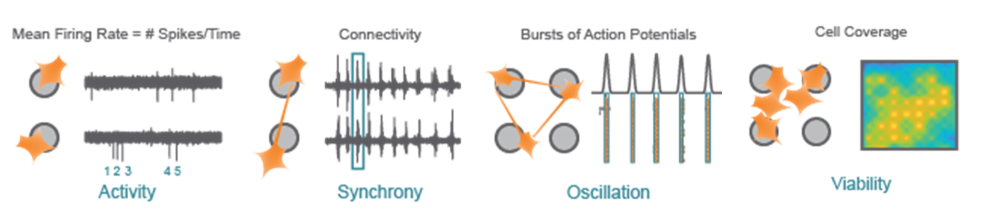
Activity - Action potentials are the defining feature of neuron function. Mean Firing Rate captures the frequency of action potentials produced by neurons.
Synchrony - Synchrony reflects the influence of functional synapses between neurons, indicating the likelihood of one neuron firing an action potential in response to another neuron.
Oscillation - Functional networks with excitatory and inhibitory neurons exhibit network-level oscillations, alternating between high and low levels of network-wide activity.
Viability - Cell coverage and viability can be monitored using impedance-based technology on the same microelectrodes used to measure neuronal function.
Network Electrophysiological Assays for Proconvulsant Assessment
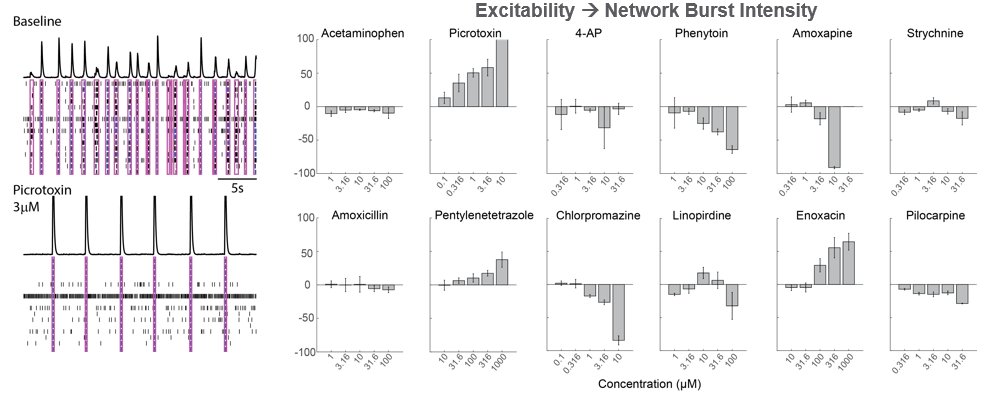
As part of the NeuTox consortium (HESI), rodent cortical neurons (Thermo Fisher Scientific) were seeded on CytoView MEA 48 well plates. At DIV28, neurons were dosed with 12 compounds at 5 doses. Network burst intensity, measured as the number of spikes per burst, changed for neuroactive compounds, increasing for most proconvulsants and decreasing for antiepileptics.
MEA Viability Quantifies Dose-Dependent Cytotoxicity
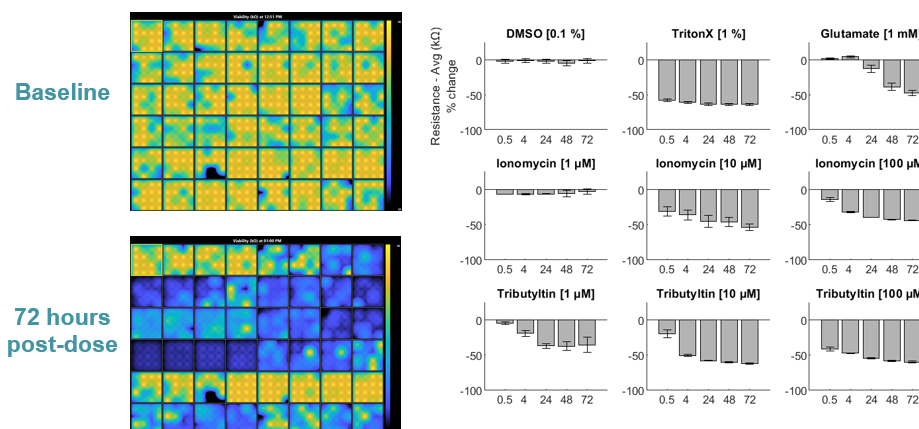
Many neuroactive compounds, such as antiepileptics and cytotoxins, can cause activity to shutdown, especially at higher doses. Measures of both cell function and viability are required to distinguish compounds that silence neural activity from those that induce cell death. Below, hiPSC-derived neurons (NeuCyte) were dosed with a variety of cytotoxins. Impedance-based MEA Viability was used to monitor cytotoxicity for 72 hrs. Because impedance is non-invasive and label-free, both function and viability can be measured repeatedly without interfering with the biology.
Multiwell MEA Technology
Microelectrode array technology
The flexibility and accessibility of neural and cardiac in vitro models, particularly induced pluripotent stem cell (iPSC) technology, has allowed complex human biology to be recapitulated in vitro. Accurate characterization the function and structure of neurons and cardiomycytes are required for a comprehensive drug safety assay.
Axion BioSystems’ Maestro™ multiwell microelectrode array (MEA) platform is a benchtop system for non-invasive functional and structural characterization of cellular networks cultured on a dense array of extracellular electrodes in each well. The Maestro records functional activity and tracks cell coverage and viability from the same microelectrodes in one simple assay.
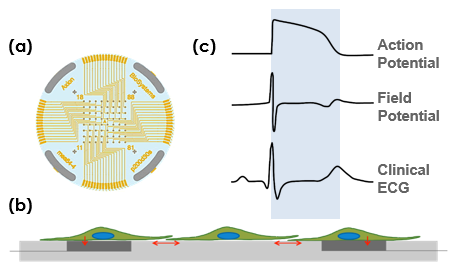
A planar grid of microelectrodes (a) interfaces with cultured neurons or cardiomyocytes (b) to model complex, human systems. Electrodes detect changes in raw voltage and record extracellular field potentials (c). The same microelectrodes can track cell viability using impedance measurements.
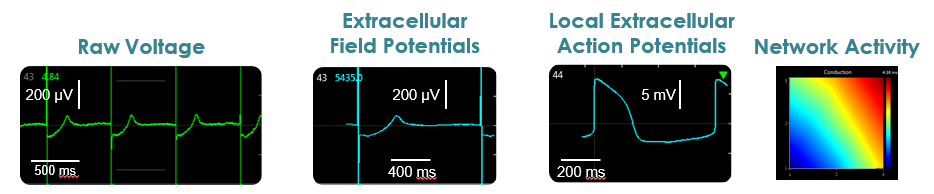
Raw voltage signals are processed in real-time to obtain extracellular field potentials from across the network, providing a valuable electrophysiological phenotype for applications in drug discovery, toxicological and safety screening, disease models, and stem cell characterization.
Introducing the Maestro Pro™ and Maestro Edge™
- Label-free, non-invasive recording of extracellular voltage from cultured electro-active cells
- Integrated environmental control provides a stable benchtop environment for short- and long-term toxicity studies
- Fast data collection rate (12.5 KHz) accurately quantifies the depolarization waveform
- Sensitive voltage resolution detects subtle extracellular action potential events
- Industry-leading array density provides high quality data from across the entire culture
- Scalable format (6-, 24-, 48- and 96-well plates) meets all throughput needs on a single system
- State-of-the-art electrode processing chip (BioCore v4) offers stronger signals, ultra-low frequency content, and enhanced flexibility