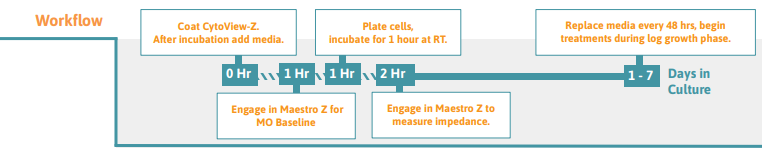
Preparing the CytoView-Z Plate
-
Pre-coat (100 µl per well is recommended) the entire well surface of the CytoView-Z plate using an extracellular matrix molecule (ECMM), such as fibronectin. Note: A working concentration of fibronectin of 1 µg/ml is recommended, however optimization of concentration or use of a different surface coating may be necessary.
-
Incubate the surface-coated plate in a cell culture incubator at 37°C and 5% CO2 for at least 1 hour.
-
Aspirate the surface coating solution from the plate.
-
Add 100 µl of complete medium to the plate, and add 8 mL of sterile water to the on-plate reservoirs to increase humidity.
-
Dock the plate in the Maestro Z to measure the media only (MO) Baseline. Transfer the plate to a biosafety cabinet when the Baseline is complete.
Culturing Adherent Cells and Transfer to CytoView-Z Plate
-
Thaw and culture the cells of interest in accordance with supplier recommendations, passaging as needed.
-
Remove flasks of cultured cells from the incubator, aspirate the media and rinse with warmed PBS. Using trypsin, or another cell dissociating agent, detach and collect the cells from the flasks as per reagent recommendations.
-
Remove a sample of the cell suspension and count the cells using a hemocytometer to determine both the cell viability and total number of viable cells.
-
Transfer the cell suspension to a 15 ml conical tube and centrifuge the cell suspension to pellet.
-
Aspirate the supernatant, being careful not to disturb the cell pellet.
-
Dilute the cell suspension in complete medium to a working concentration of cells per 100 µl. The working concentration should be the number of cells per well necessary to achieve 100% confluence within 24 hours. Note: It is recommended to run a cell density sweep with a cell type first to determine the optimal number of cells for a working concentration and to inform future experiments.
Plating Adherent Cancer Cells onto the CytoView-Z Plate
-
Undock the CytoView-Z plate from the Maestro Z once the MO baseline has been collected, and transfer the plate to a biosafety cabinet.
-
Transfer the cell suspension to a trough for easy access by a multichannel pipette. Alternatively, divide the cell suspension evenly into microcentrifuge tubes that can be used with a multichannel pipette, providing enough volume to seed the cells with a 100 µl addition per well. Tip: Gently mix the cell suspension before any addition to ensure even distribution of the cells. Dispense the cells directly in the middle of the well with the pipette tip below the surface of the liquid.
-
After seeding cells on the plate, leave it to rest in the biosafety cabinet for 1 hour at room temperature. Tip: High well-number microtiter plates are sensitive to thermal gradients, which can cause edge effects
-
Dock the plate and impedance measurements will begin automatically upon plate engagement. 16. For optimal cell health, 50% of the media should be changed after 48 hours.
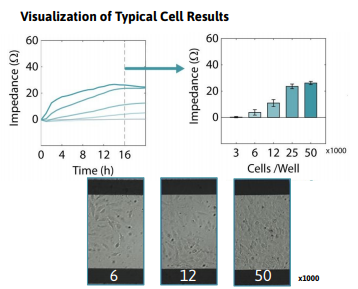
Required materials
Consumables
| Item | Vendor |
|---|---|
| CytoView-Z Plate | Axion BioSystems |
| Fibronectin | Roche |
| Dulbecco’s PBS without Ca2+/Mg 2+ | ThermoFisher |
| Kimwipes | Various |
| Trypsin or similar cell detachment agent | Various |
| 15 mL and 50 mL Centrifuge Tubes | Various |
| Pipette Tips | Various |
Equipment
| Item | Vendor |
|---|---|
| Maestro Z | Axion BioSystems |
| AxIS Z | Axion BioSystems |
| Microscope | Various |
| 1 mL Micropipettor | Various |
| 8- or 12-channel Multiwell pipettor | Various |