High Throughput Assessment of Barrier Function
Trans-Epithelial Electrical Resistance (TEER)
Epithelial and endothelial cells form barriers in the body. The strength and integrity of these barriers can be assessed via measurements of the electrical resistance across the cell layer, called TEER.
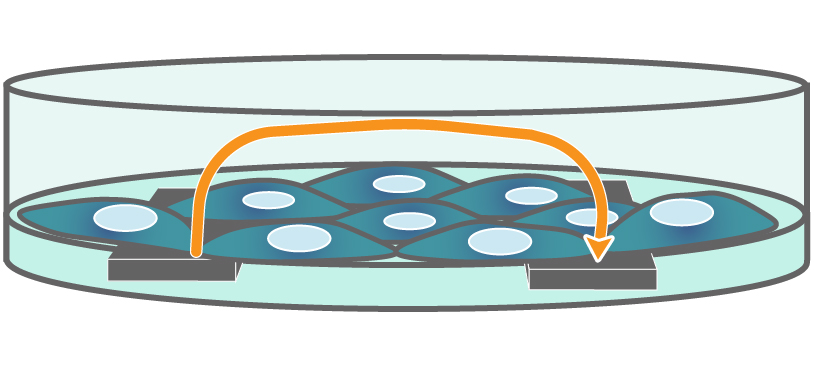
Electrodes embedded in the cell culture substrate at the bottom of each well detect small changes in the impedance of current flow. Barriers, such as tight junctions between cells, resist current flow, leading to an increase in TEER measurements of barrier function.
TEER Made Simple and Reliable
Axion BioSystems’ Maestro Z system measures cell coverage and TEER, simultaneously, across 96-wells using label-free technology. With built-in environmental controls, automated acquisition, and intuitive software controls, TEER measurements have never been easier.
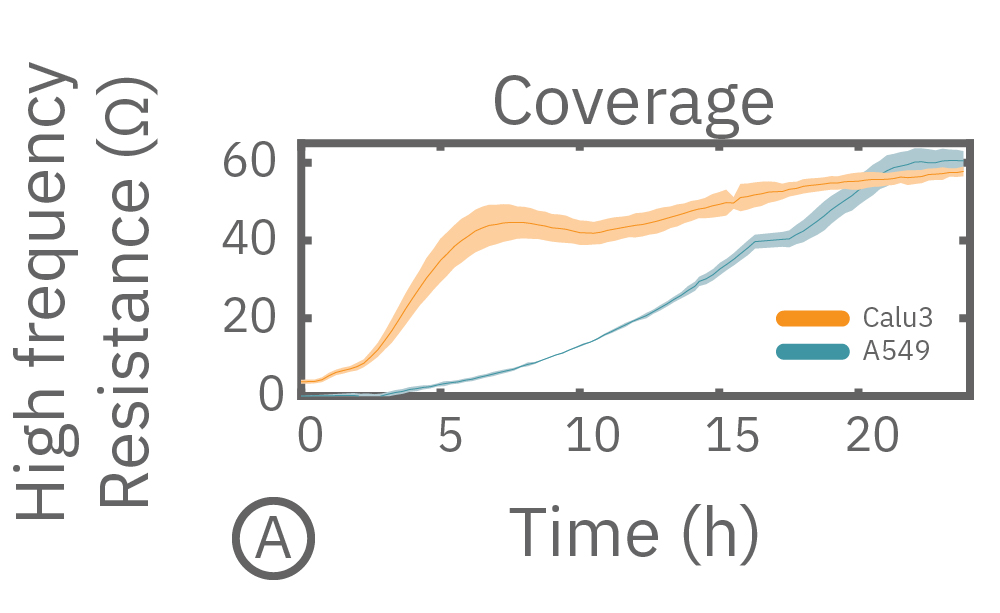
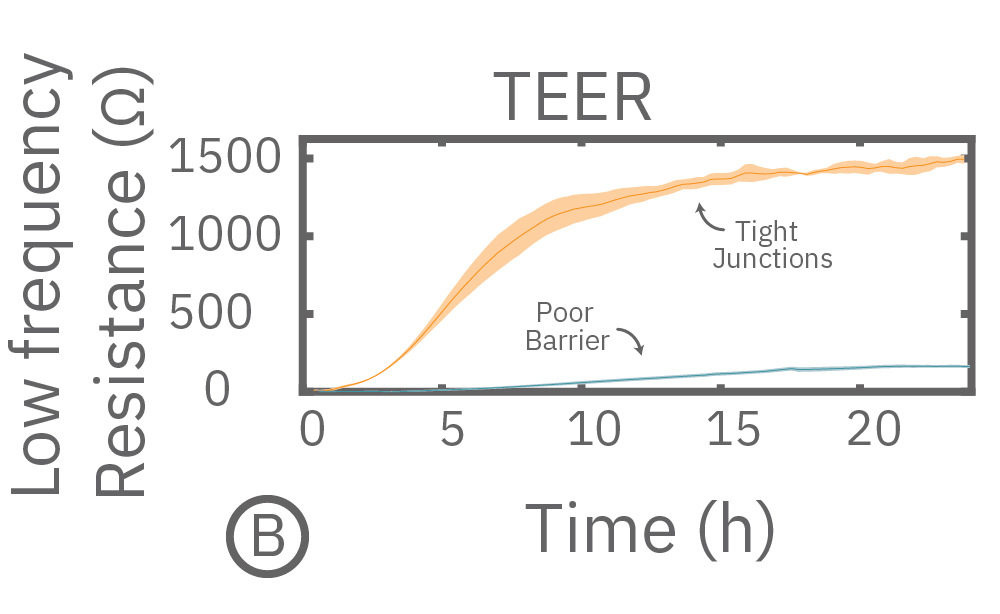
(A) Coverage, measured as resistance at 41.5 kHz, increases over time for both Calu-3 and A549 cells. (B) TEER, measured at 1 kHz, reveals that only Calu-3 cells form a strong barrier though, as they express tight junctions to block flow between neighboring cells.
The Maestro Advantage
>> Label-free, non-invasive impedance recording for long term monitoring.
>> It’s easy and hands-free! Just culture your cells in the CytoView-Z plate and measure barrier function for minutes to days.
>> Axion’s 96-well CytoView-Z plates facilitate high throughput data collection and viewing of your cells under the microscope.
>> Environmental controls provide a stable environment for both acute and long-term studies.
>> Reliable measurements from well-to-well performed simultaneously each minute.
>> Experiment management is automated via barcode tracking to simplify your assay.

TEER Measurements track barrier integrity in real-time
The Maestro Z continuously monitors cell proliferation and TEER to quantify the development of barrier properties over time. In addition to long-term monitoring, TEER on the Maestro Z is sensitive to small, transient disruptions to barrier function. Many signaling molecules in the body, such as inflammatory agents like histamine, and common drugs, like ethanol, can alter barrier permeability. Here, TEER was monitored continuously for Calu-3 cells in a 96-well CytoViewZ plate on the Maestro Z. After reaching confluence, cells were dosed with Cytochalasin D and Ethanol. Cytochalasin D inhibits actin polymerization to increase tight junction permeability, as reflected by the rapid decrease in TEER. Ethanol acts more slowly on ZO-1 and Claudin-1, two key tight junction proteins. The rapid disruption, as well as the mechanistically distinct dynamics, are captured by TEER measurements on the Maestro Z.
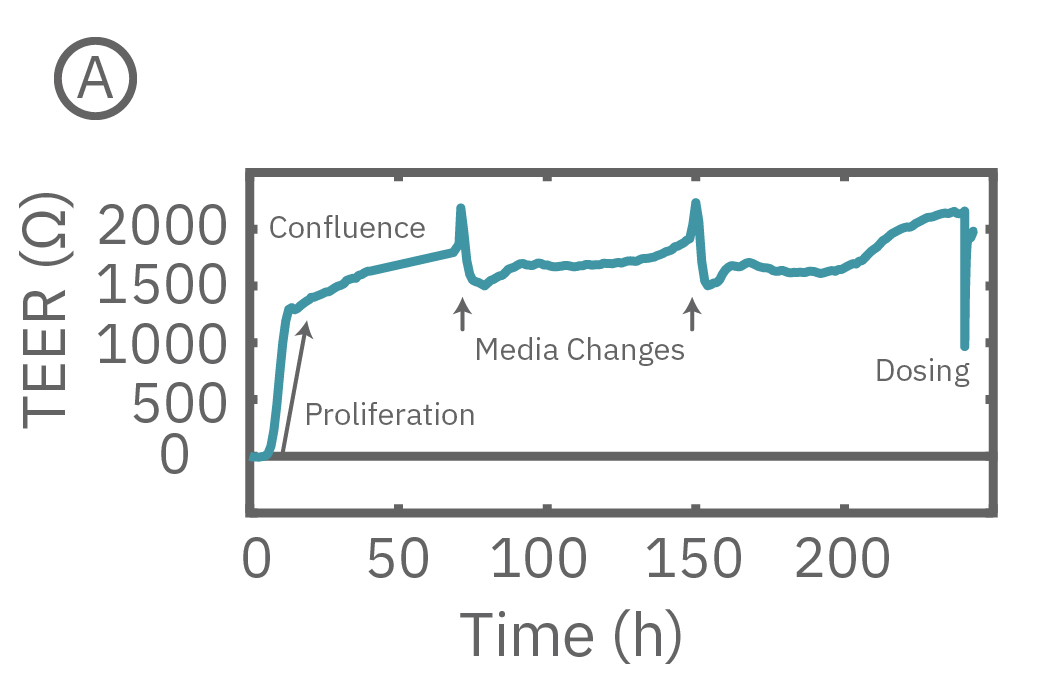
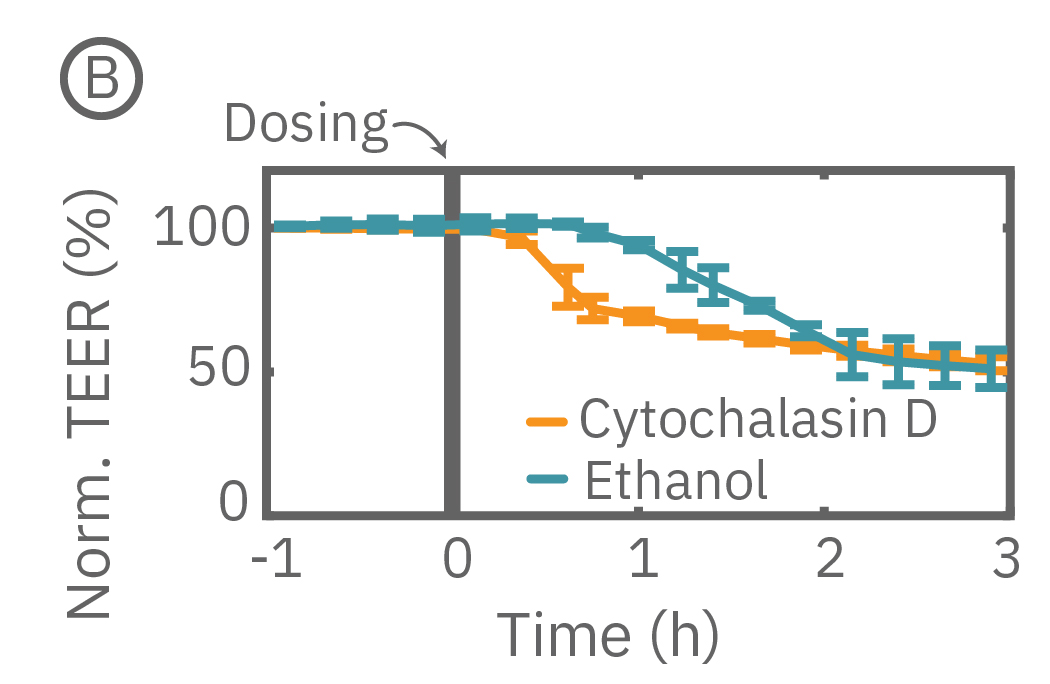
(A) TEER can be measured continuously, from proliferation to confluence, or allowed to mature in an incubator for processing of multiple plates in parallel. (B) Calu-3 monolayers were cultured for 14 days in the CytoView Z 96-well plate and then treated with Cytochalasin D and Ethanol, both of which significantly reduced the TEER.
Evaluate barrier function in disease-in-a-dish models
Barrier properties serve critical functions throughout the human body, and may be disrupted in human diseases, such as cystic fibrosis. Calu-3 is one the few immortalized cell lines that produce tight junctions and express functional cystic fibrosis transmembrane conductance regulator (CFTR), which, upon β-adrenergic stimulation, allows ion transport across the cell membrane [Shen et al., 1994]. Here, TEER measurements quantify the function of CFTR, which opens conducting ion channels to facilitate mucus secretion, a process disrupted in cystic fibrosis.
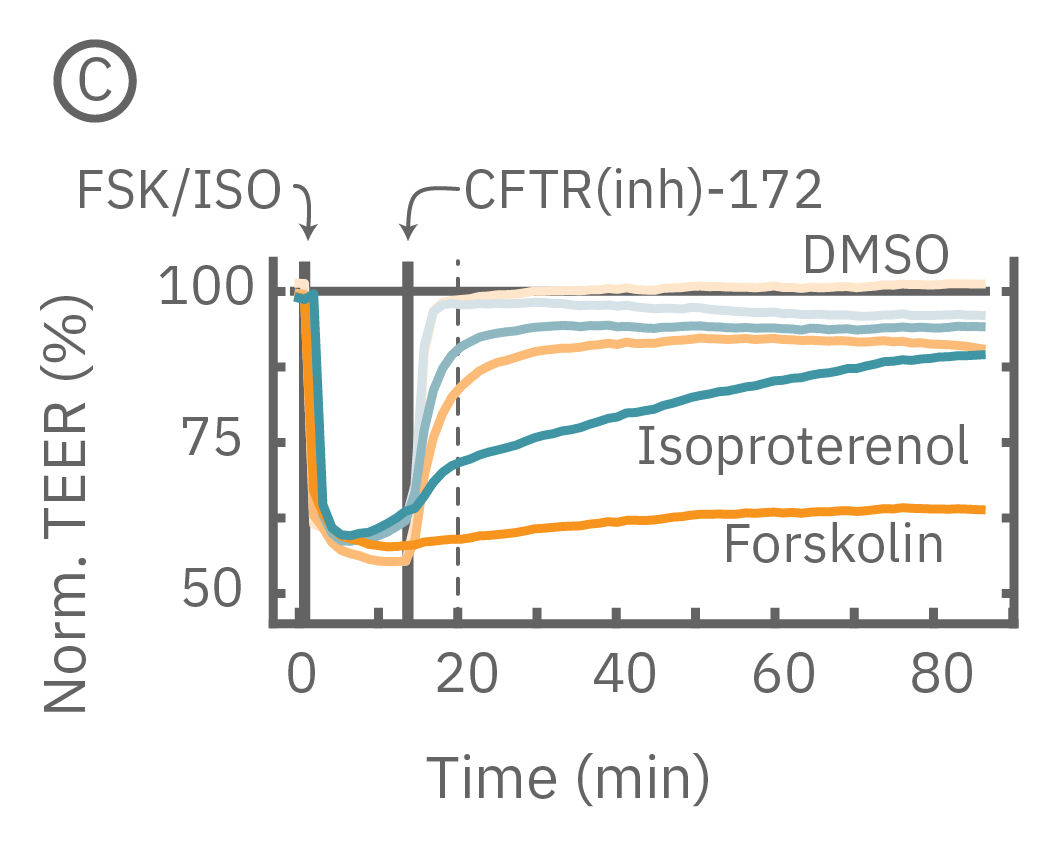
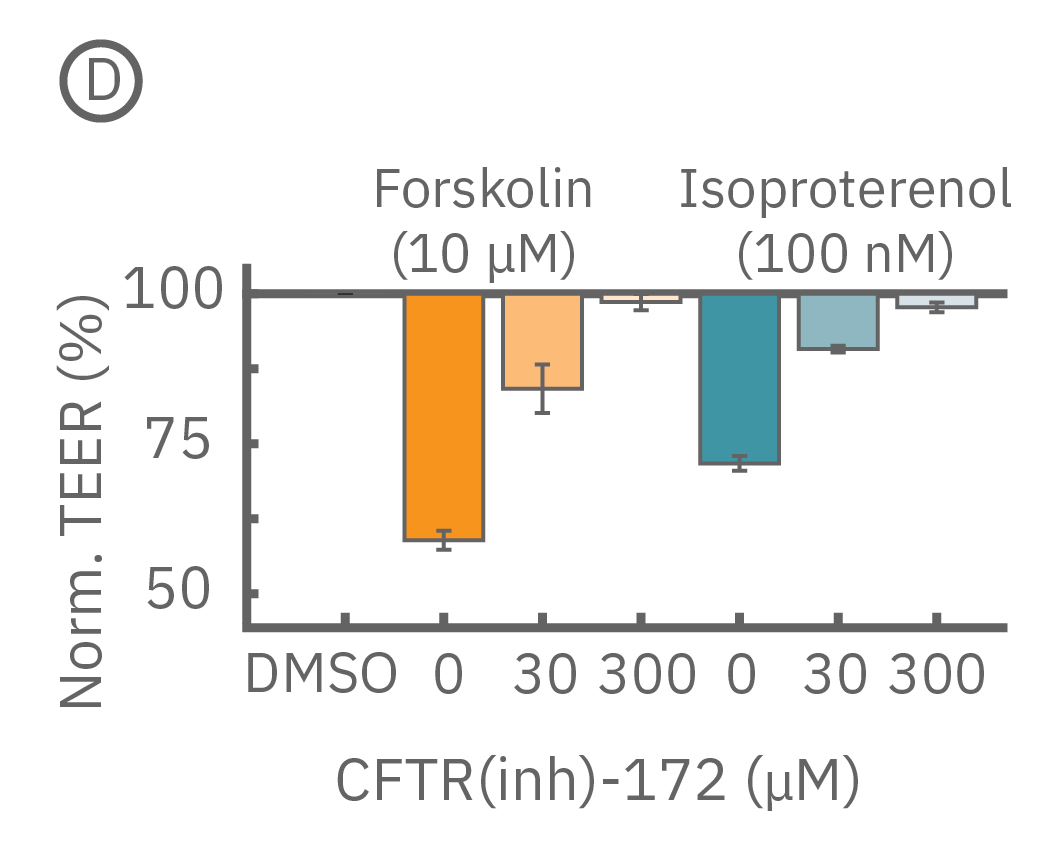
(C) Addition of isoproterenol (teal, 100 nM) and forskolin (orange, 10 µM) significantly reduced TEER of the Calu-3 cells. CFTR(inh)-172 was then added to the plate at 30 and 300 µM causing a rapid dose-dependent reversal of the isoproterenol and forskolin response. (D) 5 minutes after CFTR(inh)-172 addition isoproterenol and forskolin responses are inhibited in a dose-dependent manner.